4. Adverse events
Positive patient identification
Misidentification of patients is an important cause of avoidable harm in all areas of clinical practice, not only blood transfusion. Over the 12 month period February 2006 to January 2007, the UK National Patient Safety Agency received 24,382 reports of patients who were mismatched to their care in some way. Table 4.1 gives examples of adverse events caused by errors in identification and factors that may cause or predispose to errors. (http://www.jointcommission.org/SentinelEvents/SentinelEventAlert/sea_10.htm).
Reliable identification of patients depends on the use of standard operating procedures and the consistent application of strict rules for the items of data used to identify patients. Staff should be supported by systems such as the use of patient wristbands, patient identity cards, or handwritten or computer generated wristbands. Electronic systems for bedside checking of administration of blood or medicines have been successfully implemented (18067511, 18482189, 18346018). Whatever methods are used, the safety of patients depends on the acceptance and use of procedures approved by the hospital authorities. All personnel involved must understand the need for constant care and attention in adhering to the approved procedures.
Minimum essential data set for patient identification
To ensure positive identification of the patient in hospital there should be a specified set of identifying information that is agreed by the authority appropriate to the hospital. This should contain the following items:
- First name
- Last name
- Date of birth
- Sex
- Unique identifying number, such as:
- Social Security number
- National Health Service number
- Hospital identity number
Extra precautions needed to avoid misidentification
Unconscious patients
There must be a system that ensures reliable identification of patients who are unconscious or whose identity is unknown, for example after an accident. This is often done by using a Unique Emergency Number. This should be attached to the patient using a wrist band or some other locally specified method that ensures that the identity number remains attached to the patient during treatment and transfer to other departments. The blood request form and the blood sample tube must be labelled with the identical information. Once the patient’s full identity is known, the blood bank and other relevant departments should be informed.
Patients of different culture and language groups
Different cultures may have their own conventions for naming individuals leading to confusion about terms such as “family name”, “surname” and “first name”. Some individuals may not know their date of birth.
Babies in neonatal unit
Often there will be several infants in the same neonatal unit who have the same date of birth and for whom only the family name or mother’s name is available.
Table 4.1 Misidentification errors: causes and consequences
|
Misidentification Errors
|
|
|---|---|
|
|
|
| Factors that may cause or predispose to errors | Adverse events cased by errors in identification |
|
|
Table 4.2 Key points in patient information
|
Key Points
|
|---|
|
|
| Positive Patient Identification Key Messages |
|
Positive patient identification: key messages (table 4.2)
The patient must be:
- Positively identified before blood samples are taken for pre-transfusion testing
- Positively identified before blood is transfused
- Asked to confirm his or her identity:
- when a blood sample is taken
- before each unit of blood component is transfused
- Identifying information must be securely attached to the patient using a method defined in local rules
- If this identification is removed it must be quickly replaced
- The unconscious or unknown patient must be given a unique emergency number
Some hospital blood banks refuse to accept or process blood sample tubes or request forms that have incomplete or inaccurate information. This has been reported to result in a significant reduction in labelling errors. As with other critical steps, procedures for patient identification must be audited at regular intervals. Documents to assist with this type of audit are provided.
Haemovigilance
In simple language, haemovigilance means an organised system for
- observing, recording, analysing and reporting when something goes wrong
- using the lessons learned to take action to avoid it going wrong again.
Haemovigilance is an important part of the quality system for transfusion. Other methods for identifying errors, adverse events and reactions include audits of practice and the investigation of complaints. (PMID 12423521)
EU legal requirements
In the EU, certain aspects of haemovigilance are legal requirements governed by Directives which define haemovigilance as
- a set of organised surveillance procedures relating to serious adverse or unexpected events or reactions in donors or recipients, and the epidemiological follow-up of donors; 2002/98 EC (downloadable from the bottom of the page).
Clinical use of blood and blood components is not a competence for the European Union. It remains under the responsibility of the Member States. Therefore the EU legal requirements are restricted to reporting serious adverse events and reactions that are related to the quality and safety of blood or blood components,
Serious adverse reaction (SAR)
- defined by EU Directive as an unintended response in a donor or in a patient that is associated with the collection or transfusion of blood or blood components and that is fatal, life-threatening, disabling, incapacitating, or that results in or prolongs hospitalisation or morbidity. 2002/98 EC
- a serious adverse reaction must be reported if it may be due to the quality and safety of blood and blood components 2005 /61/EC (downloadable from the bottom of the page).
Serious adverse event
- defined by EU Directive as an untoward* occurrence associated with the collection, testing, processing, storage and distribution *of blood and blood components that might lead to death or life-threatening, disabling or incapacitating conditions for patients or which results in, or prolongs, hospitalisation or morbidity. 2002/98 EC (downloadable from the bottom of the page).
- a serious adverse event must be reported if it may affect the quality or safety of blood and blood components 2005/61/EC (downloadable from the bottom of the page).
For the different adverse reactions the EU Directive uses the International Society of Blood Transfusion (ISBT) definitions of transfusion reactions (http://www.ihn-org.net/Portal.aspx).
National haemovigilance systems
Serious adverse reactions and serious adverse events must be reported to the Competent Authority of each member state according to the procedures that it has specified,.
- adverse events and reactions that are due to problems in any part of the clinical transfusion process as defined in this Manual are not subject to mandatory reporting under the Blood Directive
- table 4.4 shows that adverse events and reactions may be due to the blood component itself, to errors in pretransfusion testing or administration, or to interactions between patient and transfused blood that may not reflect any error and that may not be preventable.
- each country may specify details of its haemovigilance arrangements that are additional to the requirements of the EU Directives. These may include a requirement to report events or reactions that are due to problems in the clinical transfusion process
* See Glossary
| Attachment | Size |
|---|---|
| 242 KB | |
| 62.6 KB |
4.1 Errors: causes, consequences and actions for quality improvement
Figures 1.2 to 1.7 in the Synopsis section of the site illustrate the causes and consequences of errors that can occur throughout the clinical transfusion process and give an outline of practical actions that can help to minimise risk.
4.2 Prevention and avoidance
The Netherlands’ haemovigilance scheme has estimated that up to half of all serious transfusion reactions are preventable by methods that are currently available.
Table 4.4 hows a classification of adverse transfusion reactions. This distinguishes (a) reactions that are due to an intrinsic quality defect in the blood component supplied (e.g. undetected hepatitis B infectivity) from (b) reactions that may result from a failure to select the correct product (e.g. irradiated components for patient at risk of GvHD) and (c) reactions, such as anaphylaxis or TRALI that may impossible to predict.
4.3 Some features of different national haemovigilance programmes
Established national haemovigilance programmes have developed somewhat different definitions and reporting requirements such as the examples that follow.
The Netherlands’ Haemovigilance Organization (TRIP), uses the term:
- Serious Transfusion Reaction – any incident that results in death or is life threatening to a patient, or that requires hospitalisation or prolongs hospital stay or that results in persistent significant disability. www.shotuk.org
A number of schemes including the UK scheme, SHOT use the term:
- Near miss – an error that might have harmed a patient but did not
National haemovigilance schemes do not all collect the same level of information, for example,
- the Netherlands’ scheme requires hospitals to report all incidences of transfusion of an incorrect blood component, but regards reporting of near misses as optional
- UK and Ireland concentrate on ‘serious hazards’ of transfusion, which are defined in their reporting schemes but do not accept reports of transfusion reactions that, although more common are considered to be less serious such as febrile non haemolytic reactions
- in France haemovigilance data is collected on all reactions regardless of severity www.ints.fr
These differences make it important to exercise care when comparing results among the different schemes. This is illustrated by the data from four national Haemovigilance schemes shown in Table 4.3, which shows very different rates of events, due in part to the different reporting requirements.
Table 4.4 Preventable and non preventable adverse events
| Type of adverse reaction | Related to the quality and safety of the supplied blood component? | Related to failure in clinical transfusion process? | Means of prevention |
|---|---|---|---|
|
Transfusion - transmitted bacterial infection |
Yes |
Possible due to failure to inspect component before transfusion |
Donor skin cleansing |
Transfusion-transmitted viral infection
|
Yes |
No |
Correct handling to avoid damage to containers Donor selection |
Transfusion-transmitted parasitic infection
|
Yes |
No |
Donor selection |
|
Haemolysis due to incorrect storage |
No |
Yes |
Quality assured clinical transfusion process |
|
Immunological haemolysis due to ABO incompatibility |
No |
Yes |
|
|
Immunological haemolysis due to other alloantibody |
No |
Yes |
|
|
Anaphylaxis or hypersensitivity |
No |
No |
May be unpredictable and unavoidable TRALI risk may be reduced with FFP from male donors |
|
Graft versus host disease |
No |
Yes |
Use of irradiated components for at-risk patients Use of amotosalen treated platelets |
|
Transfusion associated circulatory overload |
No |
Yes |
Avoid over-infusion. |
Figure 4.1 Types of adverse events and reactions. Serious hazards of transfusion (SHOT) UK.
Cumulative numbers of cases reviewed 1996-2008 n=5374
*New categories for 2008
Table 4.3 Adverse events and reactions: reported rates in different countries
|
International Comparison
|
|||
|---|---|---|---|
| Country | Status | Captures | Reports/1000 units |
| France (2005) | Mandatory | all | 2.8 |
| UK (2005) | Voluntary | serious | 0.20 |
| Ireland (2005) | Voluntary | serious | 1.22 |
| Netherlands (2006) | Voluntary | all | 2.9 |
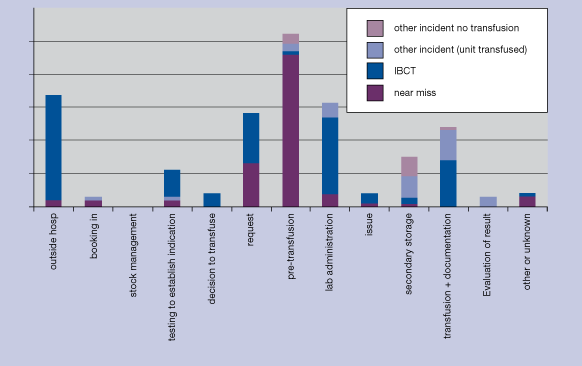
Risk management involves recording information on when errors were made, whether they were detected and how they were detected, and the reason for the error. This is sometimes called “root cause analysis”. Figures 4.2 and 4.3 show how one scheme has used its data to map the site of the first error the step in the clinical transfusion process where it occurred. In this example, the large number of incidents reported and categorised as ‘pretransfusion testing’ is mainly due to errors in collecting the pretransfusion sample rather than to errors in the blood bank laboratory. Nearly all these reports are of near misses. The corrective measure adopted in this case was to require that the blood group is always determined on two independent samples before compatible blood is issued.
Figure 4.2 Where adverse events and reactions occur in the clinical transfusion process: Netherlands haemovigilance scheme (TRIP)
Figure 4.3 Site of first error leading to potential ABO incompatibility incident Netherlands haemovigilance scheme (TRIP)