6. Blood components
This section provides a brief description of the main blood components. Full details of the specifications of blood components should be available from each blood establishment, which will have quality assurance procedures to maintain compliance with the approved specification. Blood establishments are regulated and inspected in accordance with the requirements of the relevant EU Directives.
Nonographs describing blood components can be found at www.edqm.eu (go to Guide to Preparation, use and quality control blood components).
6.1 Preparation of blood components
Until the late 1970s, most blood was transfused without being further processed to separate plasma or platelets. This was termed ‘whole blood’. Current practice in many EU countries is to process most or all whole blood donations into components – red cells, platelets and plasma.
In a typical blood establishment process, 450-500 ml of the donor’s blood is drawn into a plastic pack containing 63 ml of an anticoagulant-preservative solution such as Citrate Phosphate Dextrose (CPD) or CPD–Adenine. The citrate binds calcium and acts as an anticoagulant, and the glucose and adenine support red cell metabolism during storage. The whole blood unit may be filtered to remove white cells, most of the plasma is removed, and an additive solution, formulated to support erythrocyte metabolism, is added to the remaining red cells.
Platelet concentrate may be prepared either from the white cell and platelet layer (the so-called buffy coat) or from platelet rich plasma. Red cells, platelets, plasma and white cells can also be collected by apheresis.
Directive 2002/98 EC lists names and specifications of red cell, platelet and plasma components. These are summarised in table 6.1 at the end of this chapter. This section of the manual provides information about some of these components that are in common use.
In the manual, the term ‘red cell unit’ is used to denote the red cells from one standard blood donation.
6.2 Blood component label
The blood component label should comply with the relevant national legislation and international agreements. Most EU countries use the international labelling system known as ISBT 128 www.iccbba.org (R5 downloadable from end of page).
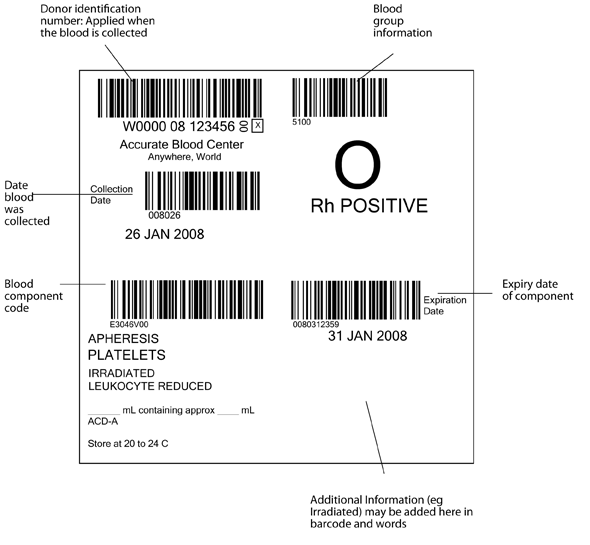
The pack label contains essential information about the blood component, as illustrated in Figure 6.1 and 6.2. The ISBT system requires that the following information be shown in barcode and eye readable form, in the four quadrants of the label.
- Top left: the unique donation number, containing a 5 digit code for the blood establishment, two digits for the year of collection, and a six digit donation number. The blood establishment name and date of the collection must be in eye readable form, (and in figure 6.1 are also shown as a barcode)
- Top right: ABO and RhD blood group
- Lower left: The identification code for the type of blood component (eg red cells, leucocyte depleted in additive solution)
- Lower right: The expiry date of the component. Additional information (eg irradiated) may be added in this quadrant in eye readable and barcode form (see fig 6.2)
Detailed information about barcoding of blood components can be found at www.iccbba.org
Figure 6.1 International ISBT 128 blood component label as specified in the ICCBBA Standard. www.iccbba.org
Directive 2002/98/EC requires that the following information should be shown on the label:
- Official name of the component
- Volume or weight or number of cells in the component (as appropiate)
- Unique numeric or alphanumeric donatior identification
- Name of producing blood establishment
- ABO Group (not required for plasma intended only for fractionation)
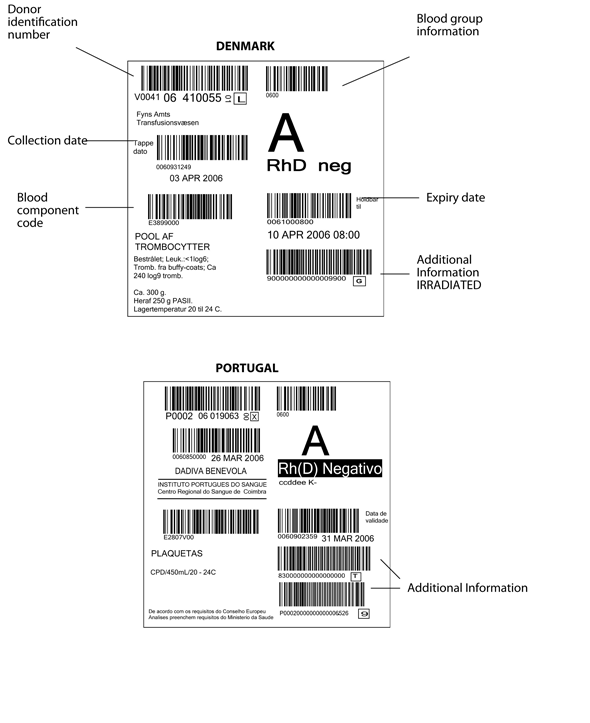
Figure 6.2 Blood component labels from EU countries
Above: Denmark, Below: Portugal
| Attachment | Size |
|---|---|
| 829.2 KB |
6.3 Labelling of blood prepared for an individual patient
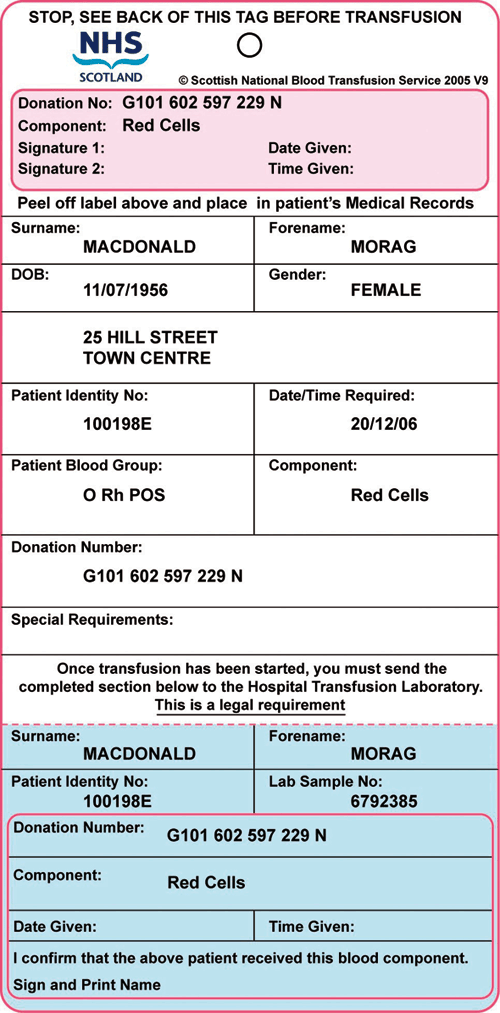
Components issued for an individual patient should also have a label that identifies the patient for whom the blood component has been prepared. This is often referred to as the Compatibility Label. It must be firmly attached to the pack and may be an adhesive label or a tie-on tag. Figure 6.3 shows an example of such a label that has been designed to provide documentary evidence of traceability.
Figure 6.3
Example of a compatibility label. It must be firmly attached to the pack and may be an adhesive label or a tie-on tag. This example can be used to provide documentary evidence of traceability.
6.4 Outline of blood component preparation and composition
This section gives a general overview of the information that clinicians may need about blood components. Other examples are given at www.edqm.eu (Search for Blood Transfusion) Guide to preparation, use and quality control of blood components) and national guides (attached at bottom of this page).
Red Cell Components
Whole blood
Typically this contains 450-500 ml of donor blood that has been collected into a pack containing 63 ml of an anticoagulant solution such as CPD.
Red cells in additive solution
Typically all but 20 ml of plasma is removed from the collected whole blood and replaced with an additive solution designed to optimise red cell preservation, such as saline solution containing added adenine, glucose and mannitol (also called SAGM, SAGMAN, Adsol or optimal additive solution). It should contain at least 45 g of haemoglobin per unit. The EU Directive refers to this as “red cells in additive solution”. Other variants of red cell components include red cells that are leucocyte-depleted, have buffy coat removed, or are collected by apheresis.
| Attachment | Size |
|---|---|
| 858.66 KB | |
| 628 KB | |
| 1.74 MB | |
| 1.55 MB |
6.5 Platelet components
Often referred to as “platelet concentrate”.
Recovered or apheresis
Platelets can be prepared by centrifuging a whole blood donation (often called recovered platelets) or collected by apheresis. Platelets prepared by each method have similar efficacy, but use of apheresis platelets exposes the recipient to the blood of fewer donors. The yield of platelets recovered from four to six whole blood donations should be 300x109 to 350x109 platelets in about 300 ml of plasma (the plasma is required to maintain platelet function during storage).
A single apheresis donation of platelets has comparable content of platelets and plasma. The use of a platelet additive solution allows platelets to be stored in reduced amounts of plasma. Platelet function is best maintained by storage at 22°C with agitation. As this temperature favours growth of some bacteria, some centres culture platelet concentrates prior to release from storage with the aim of reducing the risk of bacterial contamination. Platelets are generally stored for up to five days and some countries permit storage for seven days with special precautions.
6.6 Plasma components
Fresh frozen plasma (FFP) is separated and frozen, usually within six to eight hours after collection, to preserve factor VIII content. Other plasma components are:
- Cryoprecipitate – this is prepared by controlled thawing of frozen plasma to precipitate high molecular weight proteins, including factor VIIIc, von Willebrand factor and fibrinogen
- Cryoprecipitate depleted plasma – this is FFP from which cryoprecipitate has been prepared, leading to reduced concentration of fibrinogen and factor VIII
Leucocyte depletion
Removal of leucocytes to a level of less than one million per component by filtration or during collection of blood components by apheresis is normal practice in a number of EU countries.
Advantages of leucodepletion include a marked reduction in alloimmunisation to HLA antigens and in the risk of infection by intracellular viruses such as cytomegalovirus. Leucodepletion of red cells may also be associated with improved outcomes in some groups of patients.
6.7 Pathogen-reduced blood components
Processes that reduce or abolish the infectivity of microrganisms in blood components offer an additional level of security against transfusion transmissible infections, including those for which screening tests are not currently available. (19304113).
Plasma
Several processes are available that have been shown to cause substantial reductions in infectivity while causing only moderate reduction in activity of fibrinogen and other plasma proteins. These processes utilise methylene blue, amotosalen, or riboflavin (single donor units), or a solvent detergent treatment (applied to pool of multiple units). An alternative approach is the use of quarantined plasma (PMID 18583192).
Platelets
Platelets pose a risk of bacterial contamination because they are stored at 22°C. Bacterial culture of platelets during the storage period is used by some organisations to minimise this risk. A process for pathogen inactivation of platelets is now CE marked and in use in several countries. A further large clinical trial of efficacy and safety is due to report its findings.
Red cells
Processes for pathogen reduction of red cell components have not completed clinical trials.
6.8 Cytomegalovirus (CMV)
Cellular blood components may result in the transmission of CMV to groups of patients at risk. Practice in many EU countries is to use leucodepleted blood components to avoid this risk. In some countries the use of blood components that test negative for CMV antibody is recommended for patients at special risk of CMV infection (PMID 11149975).
6.9 Transfusion Associate Graft versus Host Disease (TA GvHD)
Transfusion can cause Graft Versus Host Disease. TA GvHD causes tissue and organ damage that is usually fatal. There is an intensive immunologic reaction mediated by transfused immunocompetent lymphocytes directed against an immunocompromised recipient, or one who shares an HLA haplotype with the donor.
The risk of TA-GVHD can be avoided by irradiation of cellular blood components or by treating platelet components with amotosalen. This inactivates T lymphocytes remaining in the component so that they are unable to engraft (PMID 18544121, 17655584, 15744235)
Irradiation may employ Gamma irradiation using a Cs137 or Co59 source, or special X-ray equipment that is now available for this purpose.
6.10 Use of washed red cells
When a patient has experienced severe allergic reactions associated with transfusion, reactions to subsequent transfusions may be prevented by the use of red cells that have been washed in sterile saline using special equipment.
This should remove residual plasma proteins cytokines or antibodies that may be the cause of the reactions. Saline washed RBCs must be used within 24 hours after washing since the saline contains no red cell nutrients and the original collection bag has been entered with consequent risk of bacterial contamination.
6.11 Clinical indications for transfusion of blood components
Summary information about the indications for use of blood components is provided in the Transfusion section of the site.
6.12 Component specifications from Directive 2004/33/EC
These are summarised in table 6.1
Table 6.1 Summary of specifcations for blood components Directive 2004/33/EC.
This table contains the information given in annex v, para 2.4
| Blood Component | Haemoglobin | Haemolysis | Leucocyte Content | |
|---|---|---|---|---|
|
RED CELLS: Volume |
||||
|
Red Cells |
Not less than 45 g per unit |
Haemolysis: Less than 0.8% of red cell mass at the end of the shelf life |
||
|
Red Cells, buffy coat removed |
Not less than 43 g per unit |
|||
|
Red Cells, leucocytedepleted |
Not less than 40 g per unit |
< 1 × 106 per unit |
||
|
Red Cells, in additive solution |
Not less than 45 g per unit |
|||
|
Red Cells, buffy coatremoved, in additive solution |
Not less than 43 g per unit |
|||
| Red Cells, leucocyte-depleted, in additive solution | Not less than 40 g per unit |
<1 × 106 per unit |
||
|
Red Cells, apheresis |
Not less than 40 g per unit |
|||
|
Whole blood Not referenced in annex V, para 2.4 of Directive 202/98 EC |
||||
|
PLATELETS: Volume Valid for storage characteristics to maintain product within specifcations for pH |
Platelet content |
pH |
Leucocyte content |
|
|
Platelets, apheresis |
Variations permitted within limits that comply with validated preparation and preservation conditions |
6,4 – 7,4 corrected for 22 °C, at the end of the shelf life |
||
|
Platelets, apheresis, leucocyte-depleted |
< 1 × 106 per unit |
|||
|
Platelets, recovered, pooled platelet-rich plasma method |
< 0,2 × 109 per single unit |
|||
|
Platelets, recovered, pooled buffy coat method |
< 0,05 × 109 per single |
|||
|
Platelets, recovered, pooled, leucocyte depleted |
< 1 × 106 per pool |
|||
|
Platelets, recovered, single unit |
< 0,2 × 109 per single unit (platelet-rich plasma method) |
|||
|
Platelets, recovered, single unit, leucocyte depleted |
< 1 × 106 per unit |
|||
|
PLASMA |
Factor VIIIc |
Fibrinogen |
Total protein |
Residual cellular content |
|
Plasma, fresh-frozen |
= / > 70 % value of the freshly collected plasma unit |
Not less than 50 g/l |
Red cells: < 6,0 × 109 /l Leucocytes: < 0, 1 × 109 /l Platelets: less than 50 × 109 /l |
|
|
Red cells: < 6,0 × 109 |
||||
|
Cryoprecipitate |
= / > 70 international units per unit |
= / > 70 140 mg per unit |
||
|
GRANULOCYTES |
Granulocyte content |
|||
|
Granulocytes, apheresis |
>1 × 1010 granulocytes per unit |
|||