7. Clinical
Optimal use of blood is defined in this manual as ‘The safe, clinically effective and effcient use of donated human blood.” However, for many of the familiar and widely accepted indications for transfusion it is a fact that there is surprisingly little high quality evidence to establish the effectiveness of transfusion therapy. As a result, clinical transfusion guidelines must often be based on inadequate information. Information in this chapter about the quality and grading of evidence for clinical practice guidelines has been drawn from the German Guidelines for Therapy with Blood Components and Plasma Derivatives (2009). Another useful sources is the database of systematic reviews at the website www.transfusionguidelines.org.uk (go to Evidence Library).
7.1 Epidemiology of blood use
The use of blood components per capita, varies widely, even among the populations of countries that have similar levels of health care. This is despite the existence of broadly similar clinic transfusion guidelines in most EU countries. This wide variation may in part be a result of differences in the demographics or patterns of disease in different populations (PMID 7889138, 19210324, 8023386). However a number of studies have shown that, at least for surgical transfusion, much of the variation cannot be explained by these factors. The low blood requirements of some surgical teams may reflect their attention to the many details of patient management that influence the need to transfuse, including the appropriate use of lower haemoglobin thresholds for transfusion, surgical and anaesthetic techniques, avoidance of hypothermia and the use of “blood sparing” technologies.
7.2 Which patients get transfused?
Studies in several European countries show that although patients undergoing surgery and treatment for malignant disease are major users of transfusion, a substantial proportion of all transfusions are used for patients who do not belong to any simple category, who are in older age groups and who have essentially “medical” conditions, often with multiple diagnoses, interventions and episodes of hospital care (PMID 19500320).
7.3 To transfuse or not?
The challenge of making an acute clinical decision about transfusion is to assess the likely benefts for the individual patient (PMID 12076437). One way to aid clinical decision-making is to use a simple checklist (downloadable from end of page>) such as the following to help focus the decision):
- What improvement in the patient’s clinical condition am I aiming to achieve?
- Can it be achieved without transfusing?
- Can I minimize blood loss to avoid the need for transfusion?
- Are there any other treatments I should give before making the decision to transfuse (such as intravenous replacement fuids oxygen, inotropes)?
- What are the specifc clinical or laboratory indications for transfusion for this patient at this time?
- What are the risks of infection or some other severe adverse event?
- Do the benefts of transfusion outweigh the risks for this particular patient?
- Will a trained person respond immediately if an acute transfusion reaction occurs?
- If this blood was for my child, or myself would I accept the transfusion or not?
- Have I recorded on the patient’s chart (and signed) my decision and my reasons for transfusion?
Decision-making can be relatively straightforward when a patient has a life-threatening major haemorrhage, bleeding associated with profound thrombocytopenia, or severe, disabling symptoms of anaemia associated with cancer chemotherapy. Indications for transfusion may also be clear in conditions such as thallasaemia or myelodysplastic disease. The decision can be much less clear – for example in an elderly patient, who has a haemoglobin concentration of 80g/l, has no evident symptoms of anaemia, is haemodynamically stable and is not bleeding.
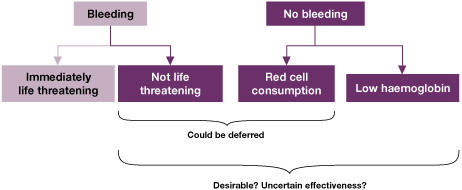
Figure 7.2 What makes us transfuse red cells
| Attachment | Size |
|---|---|
| 51.08 KB |
7.4 Urgent and emergency transfusion – major bleeding
A single patient with catastrophic bleeding can be a major challenge for the clinical and blood bank teams. When blood is required very rapidly it is extremely important to have clear communications between clinicians and the blood bank. Clinical and blood bank experience indicates that delays in providing blood in a life-threatening emergency can occur for various reasons and contribute to mortality in critical situations such as obstetric haemorrhage. www.cmace.org.uk
Hospitals should have a Major Haemorrhage Procedure that identifes roles, responsibilities and communication routes. (Example downloadable from end of page)
There should also be a clinical transfusion guideline for management of major bleeding. (example: PMID 17107347, 19135193)
Rehearsals (‘fre practices’) to familarise medical, nursing, laboratory and transport staff and to test the procedure.
Following road traffc accidents and other disasters several unconscious injured patients may arrive at the hospital within a short period, creating risks due to problems in identifying the patients. These are situations when it is vital for all the team to know and use the Major Haemorrhage Procedure (table 7.1).
Table 7.1 Example of a Major Haemorrhage Procedure
There should also be a clinical transfusion guideline for management of major bleeding.
|
Major Haemorrhage Procedure
|
||||
|---|---|---|---|---|
| Example of a Major Haemorrhage Procedure | ||||
|
||||
|
||||
|
||||
|
||||
|
||||
|
||||
|
||||
|
||||
|
||||
| Attachment | Size |
|---|---|
| 44.48 KB |
7.5 Clinical conditions that require a clinical transfusion guideline
Examples are given of procedures where possible and thses are currently used in hospitals in countries participating in the project.
Table 7.2 Clinical situations for which there should be transfusion guidelines
|
Major Haemorrhage Procedure
|
|
|---|---|
| Situation | Use this space to note reference to local clinical transfusion guideline or relevant example |
|
Blood ordering and supply in major haemorrhage |
|
|
The management of major haemorrhage in |
|
|
|
|
|
|
|
|
Critical Illness (transfusion in the intensive therapy unit) |
|
|
Preoperative assessment and optimisation |
|
|
Predeposit autologus blood – collection and transfusion |
|
|
Management of preoperative patients on drugs that affect haemostasis, such as
warfarin, heparin, clopidogrel
|
|
|
Peri-operative blood management and blood saving techniques/drugs |
|
|
Inherited coagulation disorders |
|
|
Acquired coagulation disorders |
|
|
Disseminated intravascular coagulation |
|
|
Thrombocytopenia and thrombocytopathy, TTP |
|
|
Prenatal and neonatal transfusion |
|
|
Haemolytic disease of the newborn: prevention and management |
|
|
Neonatal: exchange, intrauterine and top-up transfusion |
|
|
Chronic anaemia due to haematological disorders |
|
|
Myelodysplasia |
|
|
Haemoglobinopathies |
|
|
Autoimmune haemolytic anaemia |
|
|
Malignant haematological disorders: bone marrow failure |
|
|
Transplantation of haemopoietic stem cells |
|
|
Management of patients refusing blood transfusion |
|
7.6 Evidence: Systematic reviews and clinical guidelines
Systematic Review
This is a a review of the literature on a topic that
- is based on comprehensive searching of all relevant sources
- uses explicit criteria to assess the eligibility and methodological quality of the studies.
- uses established methods to assess the eligibility and methodological quality of the results
- may involve bringing together the of the results of several comparable studies to increase the strength of the conclusions that can be drawn (sometimes called meta analysis)
Systematic reviews relevant to transfusion can be found at www.transfusionguidelines.org.uk.
The Cochrane Library at http://www3.interscience.wiley.com is a comprehensive source of clinical trial reports and systematic reviews.
Clinical Guideline
Many important aspects of transfusion practice do not have a firm basis of evidence from well conducted randomised controlled clinical trials that have the capability to identify the most effective process or treatment. As a result, clinical guidelines often must be based on the best available information such as observational studies, case reports and properly developed concensus of professional opinion.
Example
The full document can be found at www.arzt.de (English transation downloadable from the bottom of the page). These guidelines were developed over several years on the basis of reviews of the current literature and show.
- How the quality (level) of the evidence was graded
- How the recommendations for practice were constructed.
| Attachment | Size |
|---|---|
| 869.92 KB |
7.7 Evidence-based recommendations for practice
The following is an extract from the 2009 guidelines of the Bundesaerzekammer (German Medical Association) www.arzt.de
Grading of recommendations
- Level 1: based on available data, the benefits to the patient of complying with the recommendation are judged by experts to outweigh the potential risk
- Level 2: if there are no definite data on the risk-benefit ratio
Grading of level of evidence
- Level A: data from large, prospective, randomised studies
- Level B: data from several prospective studies with conflicting results or with methodological flaws
- Level C: data from case reports and non-randomised studies.
- Level C+: data from case reports and non-randomised studies are unambiguous and confirmed by several investigations
Consequences of the recommendations
Both the level of evidence based on underlying data and the level of recommendation, reflecting the risk-benefit ratio impact on recommendation for medical practice (table 7.3).
Table 7.3 Classification of recommendations for clinical transfusion guidelines
Reproduced from: cross sectional guidelines for therapy with blood components and plasma derivatives, 4th revised edition 2009. Bundesaertztekammer (German Medical Association)
| Level of recommendation | Risk-benefit ratio | Level of evidence | Assessment of the methodological validity of the underlying data | Overall assessment, classification | Implications | Key words |
|---|---|---|---|---|---|---|
| 1 | Unambiguous | A | Randomised, controlled studies without essential methodological flaws with unambiguous results | 1 A | Strong recommendation. Valid for most patients |
shall |
| 1 | Unambiguous | C+ | No randomised, controlled studies, but unambiguous data available | 1 C+ | ||
| 1 | Unambiguous | B | Randomised, controlled study with methodological flaws. Despite unambiguous results of the study, it cannot be safely ruled out that methodical flaws have influenced the results | 1 B | Strong recommendation. Probably valid for most patients |
|
| 1 | Unambiguous | C | Observational studies without control group, but with convincing results | 1 C | Medium-strong recommendation, seems to be plausible, may be changed once improved data becomes available |
Should |
| 2 | Ambiguous | A | Randomised, controlled study without methodological reservations, but with conflicting results | 2 A | Medium-strong recommendation, depending on the individual case, a different course of action may be indicated. The interpretation of results by the Working Group Guidelines are taken into account in the recommendation |
|
| 2 | Ambiguous | C+ | No randomised, controlled studies, but data can be extrapolated from other studies | 2 C+ | Weak recommendation, depending on the individual case, a different course of action may be indicated. The interpretation of results by the Working Group Guidelines are taken into account in the recommendation |
Can |
| 2 | Ambiguous | B | Randomised, controlled study with severe flaws | 2 B | Weak recommendation, depending on the individual case, a different course of action may be indicated |
Can |
| 2 | Ambiguous | C | Observational studies, case reports | 2 C | Very Weak recommendation, depending on the individual case, a different course of action may be indicated |
Could |
| Cross sectional guidelines for therapy with blood components and plasma derivatives, 4th revised edition 2009. Reproduced with permission from Bundesaertsekammer (German Medical Association). | ||||||
7.8 Key points about the clinical indications for transfusing blood components
Red Cells
Major haemorrhage
For patients who are shocked and anaemic red cell transfusion to increase the circulating red cell mass can relieve clinical features that are caused by insufficient oxygen delivery (PMID 14556774, 9504576). Circulating blood volume must be corrected with other fluids (PMID 15163774, 12535407, 11279761). Mortality rates are high in patients who do not receive blood.
Acute anaemia
A randomised trial in ICU patients suggested that transfusion of red cells to achieve a higher haemoglobin concentration target appears to offer no benefit over more conservative transfusion to achieve a lower target Hb concentration. The exception to this may be patients with cardiovascular disease. Table 7.4 shows a recent evidence-based national clinical transfusion guideline for transfusion of red cells in acute anaemia
Germany Haemotherapy Guidelines downloadable from the bottom of the page.
Table 7.4 Evidence based national clincal transfusion guideline for transfusion of red cells in acute anaemia
Reproduced from: cross sectional guidelines for therapy with blood components and plasma derivatives, 4th revised edition 2009. Bundesaertztekammer (German Medical Association)
| The decision on transfusion for each patient should take account of that patient’s haemoglobin (Hb) concentration, capacity to compensate for acute anaemia, and risk factors | |||
|---|---|---|---|
| Hb concentration alone is not an adequate measurement of oxygen supply. If the patient is hypovolaemic the Hb concentration does not correctly reflect the red cell mass in an individual patient and it may be necessary to deviate from the recommendations below | |||
| range of haemoglobin concentration | capacity to compensate: risk factors | recommendation on red cell transfusion | strength of recommendation * |
| < 6 g/dl (3.7mmol/l) | Yes | 1C+ | |
| > 6-8 g/dl (3.7-5 mmol/l | adequate compensation: no risk factors | No | 1C+ |
| limited compensation: risk factors such as coronary artery disease, cardiac insufficiency, cerebrovascular insufficiency | Yes | 1C+ | |
| symptoms of anaemic hypoxia or decompensation (physiologic transfusion trigger) eg tachycardia, hypotension, ECG ischemia, lactic acidosis | Yes | 1C+ | |
| > 8-10 g/dl (5.0-6.2 mmol/l | symptoms of anaemic hypoxia or decompensation (physiologic transfusion trigger) e.g. tachycardia, hypotension, ECG ischemia, lactic acidosis | Yes | 2C |
| > 10g/dl (6.2 mmol/l) | No | 1A | |
Neonatal ICU patients
Transfusion of red cells to achieve a higher haemoglobin concentration target in patients who require transfusion appears to offer no benefit over more conservative transfusion to achieve a lower target Hb concentration (PMID 19117884). Target haemoglobin levels used in the key randomised controlled clinical trial depended on the age and condition of the infant.
Thalassaemia major
In countries where thalassaemia is still prevalent, it can account for a large proportion of the clinical requirement for red cell transfusion. In many countries, as a result of successful prevention programmes, most cases are now in older individuals. Red cell transfusions are typically given at two to four weekly intervals to maintain a mean Hb around 12g/dl. The aim is to fully relieve the symptoms of anaemia and suppress the patient’s own increased abnormal red cell production in the marrow (ineffective erythropoiesis). This is the cause of the skeletal abnormalities and spleen enlargement seen in under-treated patients. All patients need iron chelation therapy to prevent progressive and ultimately fatal organ damage (PMID 18413891).
Symptomatic anaemia patients with haematological malignancies or solid tumours:
The local clinical management protocol should define the range within which a patient’s haemoglobin should be maintained. A suggested arbitrary guide is to maintain Hb at not less than 9.0g/dl. As a result of complications associated with the use of erythropoetin in patients with cancer, guidelines in a number of countries now discourage or restrict its use in this situation (PMID 16705125).
| Attachment | Size |
|---|---|
| 869.92 KB |
7.9 Platelets
The normal range for the platelet count in peripheral blood at all ages is 150-400 x 109/l. A platelet count below this level does not in itself indicate a need for platelet transfusion. Isolated thrombocytopenia, in the absence of any other abnormality, is unlikely to be complicated by serious spontaneous haemorrhage if the count remains above 5 -10 x 109/l. Recent studies indicate that the clinically stable patient is unlikely to benefit from prophylactic platelet transfusion if the count is greater than 10 x 109/l. A higher threshold for transfusion is generally advised in the presence of sepsis. However, some experts question the usefulness of the platelet count in the peripheral blood as a guide to the risk of bleeding or as a means for assessing the effect of platelet transfusion.
Clinical transfusion guidelines for platelet transfusion usually cover the management of bleeding during surgery or patients with bone marrow suppression and the prevention of bleeding in patients with low platelet count due to bone marrow suppression or other causes (PMID 19109560, 15495093, 15584985, 16351634). Some guidelines specify target platelet counts. In clinical practice the recommended target platelet counts may not be achieved even with large doses of platelets.
The following is an extract from the 2009 Guidelines of the German Medical Association
Major haemorrhage:
- Transfuse if count <50 × 109/l, or
- In multiple or CNS trauma < 100 × 109/l (recommendation level 2C)
Thrombocytopenia due to chemotherapy
- Transfuse if count <10 × 109/l if not bleeding and no other risk factors (recommendation level 1A)
- Transfuse if count <20 × 109/l if at risk due to sepsis, antibiotics, abnormal clotting (recommendation level 2C)
- Transfuse if there is evident bleeding (recommendation level 1C)
Invasive surgical procedures
- Transfuse if count <50 × 109/l: < 70-100x109 in procedures, such as neurosurgery, where bleeding carries higher risks (recommendation level 1C)
Invasive diagnostic interventions
- Guidance depends on individual procedure, patient risk factors for bleeding, and risk to patient if bleeding occurs
7.10 Fresh Frozen Plasma
Although FFP is widely used, there are few well-founded indications. A systematic review of all randomised trials of FFP indicates that most clinical indications for FFP that are often recommended by transfusion practice guidelines are not supported by evidence from randomised trials.
Typical clinical guideline for plasma transfusion
Major haemorrhage
Coagulopathy with a prothrombin time prolonged > 50% is likely after replacement of 1-1.5 blood volumes. Initial dose of FFP 15-20 ml/kg. Further doses only if bleeding continues and guided by PT and APTT (1C)
Thrombotic thrombocytopenic purpura (TTP)
Plasma exchange with FFP is effective in many cases (recommendation level 1A).
Other indications
Replacement of coagulation factor deficiency, if the appropiate plasma derivative or recombinant product is not available.
7.11 Fibrinogen replacement
In many EU countries, a fibrinogen product made by plasma fractionation is used for fibrinogen replacement in dysfibrinogenaemia and acquired hypofibrinogenaemia seen in
massive transfusion and DIC. An alternative is cryoprecipitate.
7.12 Frequently asked questions about blood components
Fresh or stored red cells for transfusion?
A much cited study suggested that transfusion of stored red cells could actually impair regional oxygenation but a recent blinded, randomised, controlled study comparing the effect of fresh versus stored leucocyte-depleted red cells on systemic and regional oxygenation in ICU patients showed no definitive evidence that fresh red cells have better oxygen delivery in critically ill patients (PMID 14758149) (See download at bottom of page). A study of the effect of acute anaemia on cognitive function in healthy subjects detected no difference in the response when haemoglobin concentrations were restored with fresh or stored autologous red cells (PMID 16645441). The TRICC clinical trial (PMID 9971864) suggested that some ICU patients maintained at a lower Hb concentration, and so receiving less transfusion, may have improved outcomes. One interpretation was that this could be associated with some adverse effect of transfusing stored red cells. Randomized trials are in hand to investigate this hypothesis. Large observational studies in cardiac surgery have also suggested poorer outcomes with longer stored red cells. At present, it remains to be conclusively shown in prospective studies whether the use of fresh red cells offers benefits for critically ill patients (PMID 12393351).
Is there a case for single unit transfusion of red cells?
It is often stated that there is no case for giving a single unit transfusion, but in some cases, a single unit may be an appropriate dose. For example in a 40 kg patient with hypoxic signs or symptoms attributed to a Hb concentration of 7g/dl, a single unit of red cells may be quite sufficient to relieve symptoms (and to raise the Hb concentration by 1-2g/dl). Use of a second unit in such a case exposes the patient to additional and unnecessary risks.
Whole blood vs a red cell component?
The concept of blood component therapy (together with the requirement for plasma for fractionation) has encouraged the widespread use of red cell concentrates in most developed countries, although in some other areas of the world, most transfusions are given as red cells (R67). The clinical experience of military surgical teams is that the early administration of plasma with red cells (in approximately equal volumes) appears to be associated with better achievement of haemostasis. Whole blood may be appropriate for a patient with acute bleeding who requires both red cells and expansion of plasma volume. In cases when disseminated intravascular coagulation (DIC) contributes to the blood loss, it may be logical to use whole blood (or leucocytedepleted whole blood) since it contains at least a part of the total dose of fibrinogen and stable clotting factors that the patient requires and could reduce the need for plasma units from other donors.
Is fresh frozen plasma safe?
Worldwide, the largest avoidable risk to patients from transfusion is probably due to the transfusion of fresh frozen plasma (FFP) for unproven clinical indications. Plasma is just as likely as whole blood to transmit viral infections (other than those that are strictly cell associated). In any area where blood safety testing may be unreliable, transfusion of FFP, unless it is pathogen reduced, can be an important source of transmission of these infections.
Is fresh frozen plasma clinically effective?
There is a poor level of evidence to support many of the traditional indications for transfusing FFP (PMID 15198745). This is reflected in the recent clinical guidelines e.g. from Germany and UK. FFP should be used only to replace rare clotting factor deficiencies for which no virus-safe fractionated plasma product is available or when there is a multifactor deficiency due to severe bleeding and DIC. Other indications for FFP are the management of thrombotic thrombocytopenic purpura (TTP) and haemolytic uraemic syndrome (HUS), in which plasma infusion or plasma exchange with FFP is effective (PMID 17266701).
Does Fresh Frozen Plasma have to be used immediately after thawing?
After thawing, the level of factor VIII falls rapidly. Factor V also falls, more slowly, but level of fibrinogen and the other haemostatic proteins is maintained. Guidelines in some countries permit the use of plasma that has been stored in the blood bank for up to 24 hours after thawing. This has the advantage that plasma can be released quickly when required for urgent management of massive bleeding. In some countries, liquid plasma (never frozen) is used.
| Attachment | Size |
|---|---|
| 1.55 MB |
7.13 Avoiding the need to transfuse: planned surgery
Table 7.5 Framework for managing the preoperative patient to minimise the need for allogeneic red cell transfusion
For background information try www.nataonline.com
| Time period | Manage haemoglobin level | Manage haemostasis | Blood salvage and transfusion |
|---|---|---|---|
| Preoperative Preadmission clinic | Assess for anaemia: diagnose and treat with haematinics and epoetin if indicated | Detect and manage haemostatic defects. Stop anti-coagulants and anti-platelet drugs if safe to do so. | Arrange for intraoperative blood salvage to be available if it is appropriate for the planned operation. |
| During surgery Surgical and anaesthetic techniques | Monitor haemoglobin, haematocrit or blood loss as a guide to red cell replacement | Keep the patient warm, as cold impairs blood clotting. Rapid haemostasis testing to guide blood component replacement. Consider use of tranexamic acid where large blood loss is expected. | Use intraoperative blood salvage |
| Post-operative Control Hb concentration, manage blood loss | SOP for post-op check of Hb when haemoglobin should be checked. Minimise blood taken for laboratory samples | SOP specifying blood transfusion thresholds and targets. SOP to trigger surgical re-exploration at specified level of blood loss. Post-operative blood salvage | |
Table 7.5 provides a simple framework managing the patient waiting planned surgery so as to minimise the need for perioperative transfusion.
The following techniques have all been developed as means of reducing transfusion requirements. While some have been shown to achieve this result there is relatively little knowledge about potential risks. A recent randomised clinical trial comparing three antifibrinolytic agents has demonstrated the importance of obtaining such evidence. (See Aprotinin, below)
Preoperative autologous blood deposit (PABD)
The patient donates one or more units of his own blood which is stored till the time of surgery. May be useful for patients for whom it is very difficult to obtain compatible red cells. May reduce use of allogeneic red cells but does not reduce total red cell use when reinfused units are taken into account.
Acute normovolaemic haemodilution (ANH)
Blood is collected from the patient immediately before surgery and reinfused during or after the procedure. Evidence indicates that the procedure does not reduce transfusion requirements.
Intraoperative blood salvage
Blood lost during surgery is collected, washed to remove plasma and debris, and reinfused.
Postoperative salvage
Blood from wound drains is reinfused with or without washing.
Inhibitors of fibrinolysis
Those currently available are tranexamic acid and in some countries epsilon-aminocaproic acid. Aprotinin, the antifibrinolytic that had been extensively used for many years has recently been withdrawn because in a large randomised trial there was excess mortality in patients receiving this drug compared with those receiving tranexamic acid or EACA (PMID 18480196, 19050037).
Erythropoietin (EPO, epoietin)
EPO is a potent stimulator of red cell production. The drug is made by genetically engineered expression of the human erythropoetin gene. It is highly effective in the anaemia of chronic renal failure. Studies in patients with malignant disease have shown an increase in cancer recurrence and mortality. The risk of hypertension and thrombosis increases if the dose raises the patient’s Hb concentration to near normal levels. Parenteral iron preparations are often used with EPO to deliver the iron required for rapid erythropoiesis (PMID 16999756).
Do these technologies reduce the need for donor blood transfusion?
Clinical trials to answer this question have been subject to systematic reviews with meta-analysis. These methods reduce the use of allogeneic transfusion but may have other consequences. For example, predeposit autologous transfusion usually increases the total amount of red cell units transfused when both autologous and allogeneic units are counted.
7.14 Informing patients
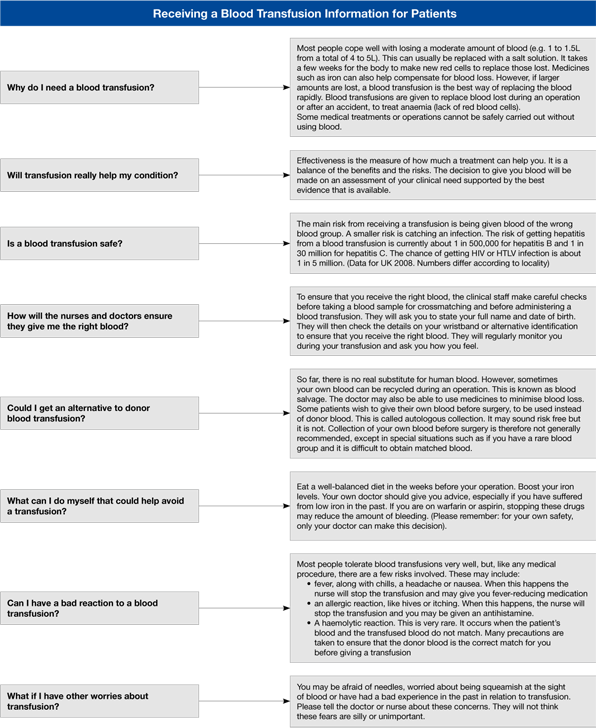
In EU member states where data are available the risks associated with receiving a transfusion are small in the context of the totality of risks of hospital care. However, as part of an effective quality system, patients who are able to communicate must be informed in good time about their treatment. Formal consent for transfusion is a requirement in some countries. Regardless of any legal requirement, the clinician has a professional duty to make sure the patient knows if and why a transfusion is required. The discussion should include the reasons why transfusion may be needed and the risks and benefits of receiving blood, (and in some circumstances of not receiving it). There are links to examples of information prepared for patients on the website.
The pre-admission clinic for elective surgery is an ideal opportunity to provide information about transfusion as part of the information given to the patient about the whole process of care. Many EU countries have information leaflets available for patients. Clinical notes should record that the patient has been given information about transfusion.
Questions frequently asked by patients
Figure 7.3 provides some information that may help in responding to questions that patients’ ask about transfusion.
Figure 7.3 Answering patients’ questions about transfusion
(for another example see http://sunnybrook.nextmovelearning.com)