4.3 Some features of different national haemovigilance programmes
Established national haemovigilance programmes have developed somewhat different definitions and reporting requirements such as the examples that follow.
The Netherlands’ Haemovigilance Organization (TRIP), uses the term:
- Serious Transfusion Reaction – any incident that results in death or is life threatening to a patient, or that requires hospitalisation or prolongs hospital stay or that results in persistent significant disability. www.shotuk.org
A number of schemes including the UK scheme, SHOT use the term:
- Near miss – an error that might have harmed a patient but did not
National haemovigilance schemes do not all collect the same level of information, for example,
- the Netherlands’ scheme requires hospitals to report all incidences of transfusion of an incorrect blood component, but regards reporting of near misses as optional
- UK and Ireland concentrate on ‘serious hazards’ of transfusion, which are defined in their reporting schemes but do not accept reports of transfusion reactions that, although more common are considered to be less serious such as febrile non haemolytic reactions
- in France haemovigilance data is collected on all reactions regardless of severity www.ints.fr
These differences make it important to exercise care when comparing results among the different schemes. This is illustrated by the data from four national Haemovigilance schemes shown in Table 4.3, which shows very different rates of events, due in part to the different reporting requirements.
Table 4.4 Preventable and non preventable adverse events
| Type of adverse reaction | Related to the quality and safety of the supplied blood component? | Related to failure in clinical transfusion process? | Means of prevention |
|---|---|---|---|
|
Transfusion - transmitted bacterial infection |
Yes |
Possible due to failure to inspect component before transfusion |
Donor skin cleansing |
Transfusion-transmitted viral infection
|
Yes |
No |
Correct handling to avoid damage to containers Donor selection |
Transfusion-transmitted parasitic infection
|
Yes |
No |
Donor selection |
|
Haemolysis due to incorrect storage |
No |
Yes |
Quality assured clinical transfusion process |
|
Immunological haemolysis due to ABO incompatibility |
No |
Yes |
|
|
Immunological haemolysis due to other alloantibody |
No |
Yes |
|
|
Anaphylaxis or hypersensitivity |
No |
No |
May be unpredictable and unavoidable TRALI risk may be reduced with FFP from male donors |
|
Graft versus host disease |
No |
Yes |
Use of irradiated components for at-risk patients Use of amotosalen treated platelets |
|
Transfusion associated circulatory overload |
No |
Yes |
Avoid over-infusion. |
Figure 4.1 Types of adverse events and reactions. Serious hazards of transfusion (SHOT) UK.
Cumulative numbers of cases reviewed 1996-2008 n=5374
*New categories for 2008
Table 4.3 Adverse events and reactions: reported rates in different countries
|
International Comparison
|
|||
|---|---|---|---|
| Country | Status | Captures | Reports/1000 units |
| France (2005) | Mandatory | all | 2.8 |
| UK (2005) | Voluntary | serious | 0.20 |
| Ireland (2005) | Voluntary | serious | 1.22 |
| Netherlands (2006) | Voluntary | all | 2.9 |
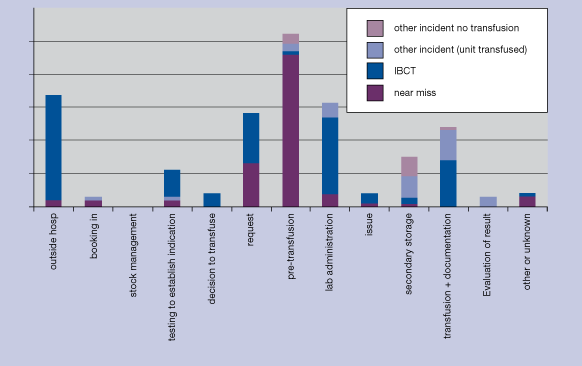
Risk management involves recording information on when errors were made, whether they were detected and how they were detected, and the reason for the error. This is sometimes called “root cause analysis”. Figures 4.2 and 4.3 show how one scheme has used its data to map the site of the first error the step in the clinical transfusion process where it occurred. In this example, the large number of incidents reported and categorised as ‘pretransfusion testing’ is mainly due to errors in collecting the pretransfusion sample rather than to errors in the blood bank laboratory. Nearly all these reports are of near misses. The corrective measure adopted in this case was to require that the blood group is always determined on two independent samples before compatible blood is issued.
Figure 4.2 Where adverse events and reactions occur in the clinical transfusion process: Netherlands haemovigilance scheme (TRIP)
Figure 4.3 Site of first error leading to potential ABO incompatibility incident Netherlands haemovigilance scheme (TRIP)