2. Purpose
To promote improvements in the quality of the clinical transfusion process, which is defined as:
Transfusion of the right unit of blood to the right patient at the right time, and in the right condition and according to appropriate guidelines.
The outcome, optimal use of blood is defined as:
The safe, clinically effective and efficient use of donated human blood
Safe: No adverse reactions or infections.
Clinically effective: Benefits the patient.
Efficient: No unnecessary transfusions. Transfusion at the time the patient needs it.
The manual is a resource for improving safety and effectiveness of the clinical transfusion process and promoting the optimal use of blood components across the EU through sharing of information and best practice.
What the Manual covers
Included
Guidance and resources to begin the development of a quality system for the clinical transfusion process.
Excluded
The collection, processing or testing of blood; blood bank technical practice; and the preparation and use of human plasma derivatives, as these products are licensed pharmaceutical products governed by other EU legislation.
The Clinical Transfusion Process in EU Countries
Details of the clinical transfusion process and its infrastructure vary among EU countries but there are essential steps that are common, shown in Figure 2.1.
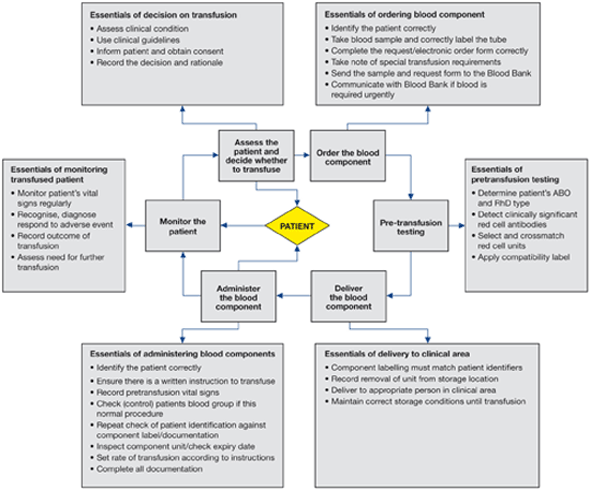
Intended audience
The manual is intended for Hospital Transfusion Committees and for medical, nursing and laboratory staff who have responsibility for patient safety and the quality of care in relation to blood transfusion. It should also be of value to other personnel who are concerned with quality improvement, risk management, accreditation, training and assessment. Patients concerned about safety of transfusion may also find it useful.
2.1 Background
Previous EU Initiatives
In 1999 the European Commission published the report “Blood safety in the European Community: an initiative for optimal use”, the outcome of a symposium held in Wildbad Kreuth, Germany. (Download from the bottom of this page.) The following paragraphs are from this report, referred to elsewhere in the text as the EU 1999 Optimal Use Initiative:
“…Considerable attention has been given to ensuring that the material collected and the processes adhered to in the preparation and distribution of (blood) products are as safe as possible. While attention has also been given to the therapeutic use … through guidelines, consensus conferences etc, there is increasing evidence that the results have been less than satisfactory and as a consequence over-use, under-use and inappropriate use of blood products persists. This can contribute to increased risks for patients and the waste of resources.”
“Transfusion of blood … involves numerous steps … which need to be strictly controlled to ensure the safety of patients and to prevent (avoidable) adverse events. These steps can be related to:
The patient, including assessment of physical condition and the need for blood under emergency or non emergency conditions; verification of identity; informed consent to the transfusion and taking a blood sample for pretransfusion testing.
The (blood) product, including reserving products in the transfusion service; identification of the assigned unit; delivery to the clinical ward and management of used and unused blood products.
The product and the patient, including identification before transfusion, administration to the patient, and documentation of outcomes.
“…Every effort should be made to establish a quality management system … in the clinical part of the blood transfusion chain.”
These points were reiterated at a second Wildbad Kreuth symposium in May 2009 on “The Optimal Clinical Use of Blood Components: Quality and Best Practices in Haemotherapy”, at which participants noted that despite many developments since 1999, concerns persist about the safety and effectiveness of blood component transfusions.
The European Commission SANGUIS study showed wide variations in surgical blood use in 43 European Hospitals during 1989-90. More recent audits have continued to show variations. Such variations in practice are an indicator of clinical uncertainty in prescribing. There were similar findings in a recent Austrian survey
| Attachment | Size |
|---|---|
| 1.22 MB |
2.2 Methods
Funding and participants
European Commission Funding for the project was obtained in spring 2007 with participants from eight countries. Participants from a further ten countries had joined the project by October 2008. In all 18 EU Member States have taken part: Austria, Czech Republic, Denmark, Estonia, France, Germany, Greece, Hungary, Italy, Malta, Netherlands, Poland, Portugal, Romania, Slovenia; and three UK countries: England, Northern Ireland and Scotland. This book and the website that supports it have been developed during a collaborative project that was initiated by the European Blood Alliance and co-funded by the European Commission and the Scottish National Blood Transfusion Service.
Project workshops
At the first project meeting in Edinburgh in May 2007 three working groups were established. The meeting, over three days, included both working group and plenary sessions. All participants, at their first project meeting, gave a short presentation on the key features of their own service and working environment. The decision to work in sequential plenary and small groups was chosen because of the degree of interaction between the topics covered by the working groups. It allowed for ideas to be developed in the small groups and then tested by discussion in the whole group. This arrangement proved successful and was continued for the subsequent workshops in Edinburgh (August 2007), Slovenia (Lake Bled) in March 2008, and Estonia (Tallinn) in October 2008. The final workshop was in Edinburgh in September 2009. Additional participants who had joined during the first year of the project were invited to form a fourth working group that has developed the Glossary for the project.
Evaluation
Participants received the first draft of the manual for the Tallinn workshop, where it was exhaustively discussed. The second draft was distributed in February 2009 with specific questions to participants. Detailed responses were received from a majority of participating countries and these were incorporated into the third draft.
Dissemination
Workshop reports, presentations, and questionnaire reports have been displayed on the project website. However it has been recognised that a website with greater functionality and capacity is essential for effective sharing and discussion of the results of the project. During the course of the project, the Project team and participants have given presentations at numerous meetings of scientific/medical societies and European bodies. An open meeting to launch the manual is planned to coincide with the 31st Meeting of the International Society of Blood Transfusion in Berlin, June 2010.
Although not funded in the EU Grant, external funds have been secured to develop the first phase of a new website that will ensure that the resources of the project are made widely available.
2.3 Language, translation and definitions
The project group worked in English. Participants recognised the challenges of achieving a shared understanding of precise meanings, especially in the case of words that may have several usages in everyday non technical language, rather than those that are specialist technical terms unique to transfusion.
The glossary is based as far as possible on definitions used in the EU Directives or taken from standard dictionaries. Where other definitions are used, the source is identified. Some key terms and definitions are also mentioned in the text.
2.4 Evidence
For many important aspects of transfusion practice, there is not a firm basis of empirical evidence that identifies the most effective process or treatment. Ideally this would be derived from well conducted, randomised, controlled clinical trials. As a result, many accepted procedures and clinical transfusion guidelines are based on the best available information and evidence, such as observational studies, case reports or professional consensus. The Transfusion section of the site provides an illustration of evidence-based practice recommendations with extracts from the 2009 German haemotherapy guidelines. (Download at the bottom of the page).
In addition, the web version of the manual provides links to the underpinning evidence where there is high quality information as judged by established grading systems. An extensive database of clinical trials and systematic reviews of evidence relating to transfusion can be found at www.transfusionguidelines.org.uk.
Evidence about common procedures in the clinical transfusion process is extremely limited and is reviewed in: PMID 18399846, 19302450 and 18194372.
| Attachment | Size |
|---|---|
| 869.92 KB |