Optimal Blood Use
The Optimal Blood Use website is a resource for improving safety and effectiveness of the clinical transfusion process, which is defined as:
Transfusion of the right unit of blood to the right patient at the right time, and in the right condition and according to appropriate guidelines.
The content of the website and the accompanying manual was developed by partners from 18 EU countries, named in the list of contributors. The manual has been translated into a number of languages.
Click on a chapter heading below to browse the English version of the manual.
1. Synopsis
Why optimal blood use is important
The safety of hospital treatment and the effectiveness of care are major concerns in healthcare systems. Blood transfusion has been the subject of legal proceedings and investigations in Canada, England, France, Ireland and other countries. Hospitals should be in a position to show that their practice of blood transfusion is safe, clinically effective and efficient. Specific reasons for this are as follows.
Accountability
Blood is a human tissue and is a precious and scarce resource. Many countries have difficulties matching supply with demand. The supply of blood components in the EU depends substantially on the support of voluntary donors. Both the ageing population in many EU countries and the effect of new precautionary measures to safeguard blood recipients have increased the problems of maintaining a sufficient supply of blood. Transfusion services promote donation as an essential contribution to the care of patients, so both hospitals and blood collection services have an obligation to demonstrate to blood donors that each gift of human tissue is carefully, wisely and effectively used and that it can be fully accounted for. Patients need assurance that blood is safe, available and used only when required.
Compliance with EU legislation
EU Blood Directives place a legal responsibility on hospital managements to introduce a quality system to important parts of the transfusion chain. Blood establishments are required, to maintain quality management systems and to undergo regular inspection.
Hospital blood banks must submit an annual compliance form and may be inspected on the basis of this return. The reporting of adverse events is a legal requirement in the EU as is the ability to trace every blood component from its donor to the patient who receives it.
Accreditation
Institutions that seek accreditation by bodies such as the Joint Commission or the Care Quality Commission in the UK will need to show evidence of a quality management system.
R42 is downloadable from files at the end of the page.
www.nhshealthquality.org
Legal and media pressures
Legal actions, public inquiries, investigations or adverse media attention stimulated by transfusion-related harm to patients are likely to gain serious management attention (and the application of resources) to avoid future problems. Experience in several countries has shown that adverse events can cause medico-legal, publicity and reputational risks for a hospital and sometimes for the wider healthcare system.
Cost
The cost of providing blood components has increased as a result of new safety requirements and other technical developments. As an example in France, the overall cost of blood components increased by 37% between 1998 and 2008. The cost per inhabitant of France was €6.8 in 1999 and €8.8 in 2008.
Summary of the manual
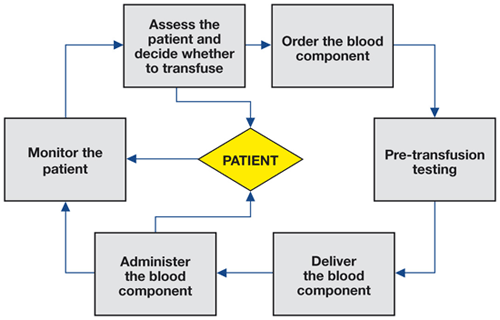
Details of the clinical transfusion process vary among the member states of the EU, but there are essential steps that are common to most. These are shown in Figure 1.1, and more detail is shown in the purpose section of this site (figure 2.1).
Quality improvement: analysis and prevention of errors
The following figures (1.2 to 1.7) share the same layout and show, for each of the main steps in the clinical transfusion process, examples of errors or failures in the process, the possible consequences for the patient, some underlying reasons for failures or errors, and finally some key points about prevention and avoidance. The subject matter of these tables is covered in more detail in the various sections throughout the site.
Figure 1.2 Analysis and prevention of errors in clinical transfusion decisions
|
Clinical Decision
|
||||
|---|---|---|---|---|
| Steps in the process | What can go wrong | Consequences for the patient |
Why it goes wrong | Prevention and avoidance |
|
|
|
|
|
Figure 1.3 Analysis and prevention of errors in ordering blood components
|
Patient Sample and Request for Blood
|
||||
|---|---|---|---|---|
| Steps in the process | What can go wrong | Consequences for the patient |
Why it goes wrong | Prevention and avoidance |
|
|
|
|
|
Figure 1.4 Analysis and prevention of errors in pretransfusion testing
|
Errors in Pretransfusion Testing
|
||||
|---|---|---|---|---|
| Steps in the process | What can go wrong | Consequences for the patient |
Why it goes wrong | Prevention and avoidance |
|
|
|
|
|
Figure 1.5 Analysis and prevention of errors in delivering blood to the clinical area
|
Deliver Blood Component to the Clinical Area
|
||||
|---|---|---|---|---|
| Steps in the process | What can go wrong | Consequences for the patient |
Why it goes wrong | Prevention and avoidance |
|
|
|
|
|
Figure 1.6 Analysis and prevention of errors in administering (transfusing) blood
|
Administer Blood Component
|
||||
|---|---|---|---|---|
| Steps in the process | What can go wrong | Consequences for the patient |
Why it goes wrong | Prevention and avoidance |
|
|
|
|
|
Figure 1.7 Analysis and prevention of errors in monitoring the transfused patient
|
Monitor the Transfused Patient
|
||||
|---|---|---|---|---|
| Steps in the process | What can go wrong | Consequences for the patient |
Why it goes wrong | Prevention and avoidance |
|
|
|
|
|
| Attachment | Size |
|---|---|
| 210.55 KB |
2. Purpose
To promote improvements in the quality of the clinical transfusion process, which is defined as:
Transfusion of the right unit of blood to the right patient at the right time, and in the right condition and according to appropriate guidelines.
The outcome, optimal use of blood is defined as:
The safe, clinically effective and efficient use of donated human blood
Safe: No adverse reactions or infections.
Clinically effective: Benefits the patient.
Efficient: No unnecessary transfusions. Transfusion at the time the patient needs it.
The manual is a resource for improving safety and effectiveness of the clinical transfusion process and promoting the optimal use of blood components across the EU through sharing of information and best practice.
What the Manual covers
Included
Guidance and resources to begin the development of a quality system for the clinical transfusion process.
Excluded
The collection, processing or testing of blood; blood bank technical practice; and the preparation and use of human plasma derivatives, as these products are licensed pharmaceutical products governed by other EU legislation.
The Clinical Transfusion Process in EU Countries
Details of the clinical transfusion process and its infrastructure vary among EU countries but there are essential steps that are common, shown in Figure 2.1.
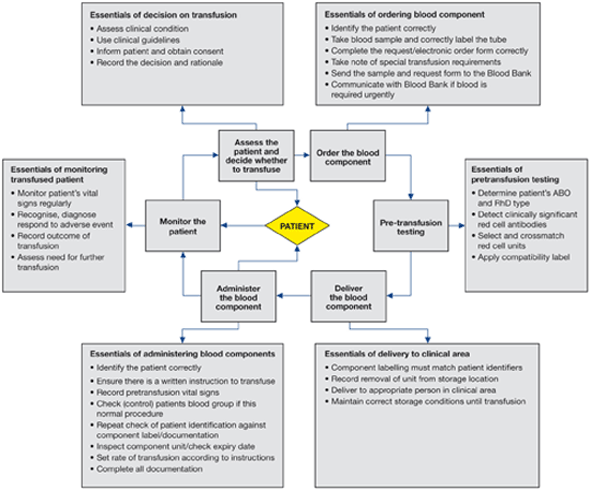
Intended audience
The manual is intended for Hospital Transfusion Committees and for medical, nursing and laboratory staff who have responsibility for patient safety and the quality of care in relation to blood transfusion. It should also be of value to other personnel who are concerned with quality improvement, risk management, accreditation, training and assessment. Patients concerned about safety of transfusion may also find it useful.
2.1 Background
Previous EU Initiatives
In 1999 the European Commission published the report “Blood safety in the European Community: an initiative for optimal use”, the outcome of a symposium held in Wildbad Kreuth, Germany. (Download from the bottom of this page.) The following paragraphs are from this report, referred to elsewhere in the text as the EU 1999 Optimal Use Initiative:
“…Considerable attention has been given to ensuring that the material collected and the processes adhered to in the preparation and distribution of (blood) products are as safe as possible. While attention has also been given to the therapeutic use … through guidelines, consensus conferences etc, there is increasing evidence that the results have been less than satisfactory and as a consequence over-use, under-use and inappropriate use of blood products persists. This can contribute to increased risks for patients and the waste of resources.”
“Transfusion of blood … involves numerous steps … which need to be strictly controlled to ensure the safety of patients and to prevent (avoidable) adverse events. These steps can be related to:
The patient, including assessment of physical condition and the need for blood under emergency or non emergency conditions; verification of identity; informed consent to the transfusion and taking a blood sample for pretransfusion testing.
The (blood) product, including reserving products in the transfusion service; identification of the assigned unit; delivery to the clinical ward and management of used and unused blood products.
The product and the patient, including identification before transfusion, administration to the patient, and documentation of outcomes.
“…Every effort should be made to establish a quality management system … in the clinical part of the blood transfusion chain.”
These points were reiterated at a second Wildbad Kreuth symposium in May 2009 on “The Optimal Clinical Use of Blood Components: Quality and Best Practices in Haemotherapy”, at which participants noted that despite many developments since 1999, concerns persist about the safety and effectiveness of blood component transfusions.
The European Commission SANGUIS study showed wide variations in surgical blood use in 43 European Hospitals during 1989-90. More recent audits have continued to show variations. Such variations in practice are an indicator of clinical uncertainty in prescribing. There were similar findings in a recent Austrian survey
| Attachment | Size |
|---|---|
| 1.22 MB |
2.2 Methods
Funding and participants
European Commission Funding for the project was obtained in spring 2007 with participants from eight countries. Participants from a further ten countries had joined the project by October 2008. In all 18 EU Member States have taken part: Austria, Czech Republic, Denmark, Estonia, France, Germany, Greece, Hungary, Italy, Malta, Netherlands, Poland, Portugal, Romania, Slovenia; and three UK countries: England, Northern Ireland and Scotland. This book and the website that supports it have been developed during a collaborative project that was initiated by the European Blood Alliance and co-funded by the European Commission and the Scottish National Blood Transfusion Service.
Project workshops
At the first project meeting in Edinburgh in May 2007 three working groups were established. The meeting, over three days, included both working group and plenary sessions. All participants, at their first project meeting, gave a short presentation on the key features of their own service and working environment. The decision to work in sequential plenary and small groups was chosen because of the degree of interaction between the topics covered by the working groups. It allowed for ideas to be developed in the small groups and then tested by discussion in the whole group. This arrangement proved successful and was continued for the subsequent workshops in Edinburgh (August 2007), Slovenia (Lake Bled) in March 2008, and Estonia (Tallinn) in October 2008. The final workshop was in Edinburgh in September 2009. Additional participants who had joined during the first year of the project were invited to form a fourth working group that has developed the Glossary for the project.
Evaluation
Participants received the first draft of the manual for the Tallinn workshop, where it was exhaustively discussed. The second draft was distributed in February 2009 with specific questions to participants. Detailed responses were received from a majority of participating countries and these were incorporated into the third draft.
Dissemination
Workshop reports, presentations, and questionnaire reports have been displayed on the project website. However it has been recognised that a website with greater functionality and capacity is essential for effective sharing and discussion of the results of the project. During the course of the project, the Project team and participants have given presentations at numerous meetings of scientific/medical societies and European bodies. An open meeting to launch the manual is planned to coincide with the 31st Meeting of the International Society of Blood Transfusion in Berlin, June 2010.
Although not funded in the EU Grant, external funds have been secured to develop the first phase of a new website that will ensure that the resources of the project are made widely available.
2.3 Language, translation and definitions
The project group worked in English. Participants recognised the challenges of achieving a shared understanding of precise meanings, especially in the case of words that may have several usages in everyday non technical language, rather than those that are specialist technical terms unique to transfusion.
The glossary is based as far as possible on definitions used in the EU Directives or taken from standard dictionaries. Where other definitions are used, the source is identified. Some key terms and definitions are also mentioned in the text.
2.4 Evidence
For many important aspects of transfusion practice, there is not a firm basis of empirical evidence that identifies the most effective process or treatment. Ideally this would be derived from well conducted, randomised, controlled clinical trials. As a result, many accepted procedures and clinical transfusion guidelines are based on the best available information and evidence, such as observational studies, case reports or professional consensus. The Transfusion section of the site provides an illustration of evidence-based practice recommendations with extracts from the 2009 German haemotherapy guidelines. (Download at the bottom of the page).
In addition, the web version of the manual provides links to the underpinning evidence where there is high quality information as judged by established grading systems. An extensive database of clinical trials and systematic reviews of evidence relating to transfusion can be found at www.transfusionguidelines.org.uk.
Evidence about common procedures in the clinical transfusion process is extremely limited and is reviewed in: PMID 18399846, 19302450 and 18194372.
| Attachment | Size |
|---|---|
| 869.92 KB |
3. Quality systems
Patients’ questions:
One way of introducing the concept of quality management in clinical transfusion is to consider some questions that any patient might ask if they believe that a transfusion may be given.
Here are some examples:
- Do I really need to have a blood transfusion?
- Will it help me?
- Could a transfusion do me harm?
- Will they give me the right blood?
- Will I feel unwell during the transfusion?
- If I start to feel bad during the transfusion will someone come to help me?
- If I need blood in an emergency will they get it to me in time?
- Will someone knowledgeable take the time to explain all this to me?
- Is the hospital staff properly trained to give me the transfusion?
- How do I know that the hospital does these things well?
With these questions, the patient is seeking some evidence that the hospital does a good job in providing blood transfusions.
One way that the hospital can give reassurance is by providing evidence that things are done correctly. This could be information about training, documentation of procedures, or results of checks of performance or comparisons of results between one hospital and others. All these are important parts of a quality system. (11234582)
This Manual provides practical guidance that can help to provide answers to questions of this type, whether they are asked by patients or, in different ways, by quality inspectors, auditors or regulators.
A quality system (QS) for the clinical transfusion process should:
- Provide assurance to patients, the community and clinicians that treatment is safe, effective and efficient, the people who carry out each step of the process know what they are doing, how to do it and why they are doing it
- Provide evidence that tasks are carried out correctly and consistently using the right procedures
- Lead to improvement in quality by providing evidence about performance and by encouraging everyone concerned to learn from both mistakes and successes
Successful introduction of a QS depends on strong management support to make sure that:
- Responsibility for developing and maintaining the QS is clearly assigned
- Sufficient staff, proper working conditions, facilities and training are provided
- An effective programme of evaluation or audit is in place
Why transfusion should be part of a hospital’s wider quality system
Many studies show that patients suffer avoidable harm due to errors and accidents (quality failures) in hospitals. These occur in many aspects of the process of care. For most patients and their clinicians, transfusion is only one element of the whole process of care and transfusion risks are only a small proportion of all the risks to which patients are exposed. For these reasons a quality management system for transfusion should be planned as part of a hospital’s wider quality system. This was a key conclusion of the 2009 Wildbad-Kreuth Symposium.
3.1 Clinical quality assurance
Quality systems have developed largely in relation to manufacturing processes. The same broad principles apply to the clinical setting. However some of the vocabulary, concepts and methods used by quality experts are unfamiliar to many clinicians and also they may not apply directly to the clinical context.
For this reason we have used simple and non-specialist terms where possible. Relevant extracts from the EU directives are shown throughout the text. One relevant definition of clinical quality assurance is:
“Improving performance and preventing problems, through planned and systematic activities including documentation, training and review.”
3.2 Quality system for clinical transfusion
Essential elements include:
Leadership
- Management demonstrates commitment to quality
- Responsibility for quality is clearly assigned
- Resources are available
- There is an effective hospital transfusion committee or equivalent
Standards or specifications
- There are explicit statements of what a product should be or a process should achieve
Documentation
- There are written instructions for doing each job
- There are records to show whether the job has been done correctly
Change control
Changes in procedures are introduced in a controlled fashion and proper records are maintained
Evaluation or Audit
- Performance is independently assessed
Staff Training and Assessment
- Staff are taught what to do and why it is important
- Their knowledge and competence is assessed
Quality Improvement
3.3 Success factors
Professional leadership
A key success factor can be leadership provided by a respected senior clinician who develops an active professional interest in improving transfusion treatment. ‘Clinical Champions’ for good transfusion may emerge from specialties such as anaesthetics/ intensive care, surgery or haematology, where transfusion is frequently utilised. One approach that has been successful is to engage such specialists in collaborative programmes of clinical audit or research on the use of transfusion in their own specialist field.
Effective Hospital Transfusion Committee
An effective and well-led Hospital Transfusion Committee (HTC) or a body with equivalent functions is widely held to be essential for improvement of clinical transfusion practice. The primary aim should be to promote a high standard of care for patients at risk of transfusion (i.e. those who must be transfused, and also those who, with good clinical management, may avoid the need for transfusion). The HTC should have a clear line of accountability to an appropriate post at a senior management level in the institution. The HTC should have the authority to determine hospital policy in relation to blood transfusion and must have an effective means of disseminating it to all relevant staff and to patients where appropriate. (www.transfusionguidelines.org.uk)
Terms of Reference for an HTC
Should include the following:
- Promote the dissemination and the use of national or local guidelines that apply to the clinical transfusion process
- Regularly review and update the hospital’s documentation for blood transfusion
- Carry out audits that evaluate the hospital’s clinical blood transfusion process against the relevant guidelines and benchmark the use of blood components against best practice
- Promote the education and training of clinical, laboratory and support staff involved in the clinical transfusion process
- Report serious adverse reactions and events to the national haemovigilance programme
- Ensure that incidents are analysed and the information is used to help improve practice and prevent a repetition
Membership of HTC
The HTC should include clinicians from specialties in the hospital that utilise transfusion, for example haematology, anaesthetics, intensive care, surgery, or obstetrics, as well as staff from nursing, blood bank and audit or research departments. The committee requires an effective chairperson who has the professional respect of senior medical personnel and can command the attention of hospital management.
Operation of HTC
The HTC should meet regularly, have a formal agenda and keep full records of its decisions. It must have the authority and support to ensure that its decisions are effectively communicated to and followed by staff who contribute to the clinical transfusion process.
Someone who is employed to make things happen
The transfusion committee may make excellent recommendations, but it needs an executive officer, a person who is employed specifically to ensure that the recommendations are converted into actions. Several countries have created a new position for this purpose. The manual uses the term Transfusion Practitioner (TP) (www.betterblood.org.uk) but posts with similar responsibilities have also been given titles such as Transfusion Safety Officer (TSO), Transfusion Nurse Coordinator (TNC) or Haemovigilance Officer (www.transfusionsafety.ca). The TP is concerned with the clinical transfusion process, ”taking quality assurance from the blood bank to the patient”. The transfusion practitioner’s job description would typically specify responsibilities such as these:
- Education and training of nursing and medical staff
- Patient information
- Promote compliance and safety in activities such as specimen collection, administration blood components and products
- Carry out transfusion practice audit
- Investigate and report adverse events and reactions
- Trouble shoot and take preventive and corrective action
- Support development and implementation of transfusion policies and guidelines
In many countries the TP have a background in nursing or the transfusion laboratory; other countries have employed doctors or pharmacists in similar roles. The goal should be for the TP to be part of a wider transfusion team that should develop with the encouragement and motivation of the transfusion committee. In several EU countries, the TP role is now viewed as an essential part of the hospital’s quality improvement programme in transfusion.
3.4 Hospital Transfusion Team
The Departments of Health in the UK have recommended that hospitals have a Hospital Transfusion Team (HTT) to manage the day-to-day business of blood transfusion within the hospital. Membership should include a medical transfusion specialist, the head of the blood bank and the transfusion practitioner.
3.5 Managing the environment
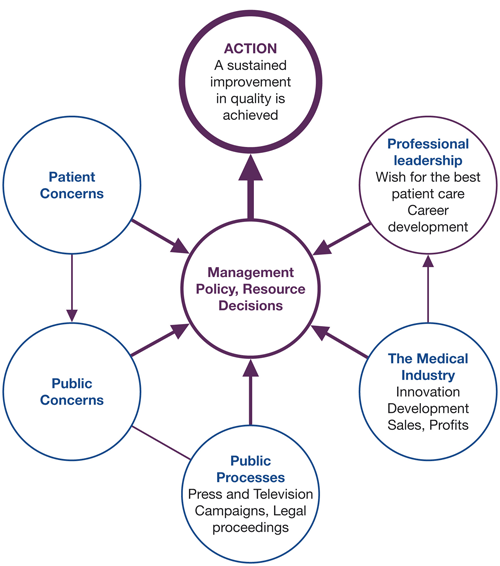
Success in creating change and improvement depends on factors other than the scientific or technical. It is important to be aware of the many influences on the ability to make change happen. Awareness of pressures such as those illustrated in Figure 3.1 can improve the ability to influence decisions and actions. Research also shows the importance of a better understanding of psychological and behavioural factors that underlie the behaviours of health care professionals.
Figure 3.1 Environmental factors affecting quality
3.6 Quality indicators for blood transfusion
Evaluation of the clinical use of blood products is often done by monitoring or surveying clinical practice against objective indicators of performance. This is perhaps better described as benchmarking than audit. Useful indicators of practice (quality or performance indicators) must be easy to collect and quantifiable.
Quality indicators may be used to monitor and evaluate the quality of the therapeutic transfusion process or compliance with clinical guidelines. There are two types of indicators: internal and external.
Internal indicators are used for quality management and improvement of the clinical transfusion process within an institution. They must be relevant for the critical steps in the process and the professionals involved. They must be specific and detailed, easy to sample, educative and effective in stimulating actions for improvement.
External indicators provide information for external control agencies such as health care inspectorate and/or for comparison between hospitals (benchmarking). These have to provide monitoring or signaling information about the quality of the process, measure global aspects such as global outcome and require good validation. According to what they measure three types of indicators can be described:
Structure indicators: How well have I organised the process?
Process indicators: Am I doing well?
Outcome indicators: Do I reach the result required?
Indicators are only one tool for evaluating practice. In some cases audit may provide better information. However, if used in the right way indicators may be an efficient and tool for improving the quality of the therapeutic transfusion process.
3.7 Specific indicators of transfusion practice
The following list is a practical example from the Leiden University Hospital in the Netherlands, where indicators are sampled annually and reviewed by the Hospital Transfusion Committee. This identifies priorities and sets targets for evaluation.
Management of hospital stock
The number of expired products in the stock of the hospital blood bank divided by the total number of blood products in the stock of the hospital blood bank.
Prescription
The number of units of blood components (red cells, platelets and fresh frozen plasma) that are not prescribed according to the known guidelines, divided by the number of prescriptions for blood products (red cells, FFP, platelets) in the same period.
Ordering and wastage
The number of blood components (red cells, platelets and fresh frozen plasma) returned to the hospital blood bank by a department, divided by the total number of blood components supplied by the blood bank service to that department.
The number of blood components that are not transfused divided by the number obtained from the blood establishment.
Request forms
The number of blood product request forms lacking essential data divided by the total number of orders for blood components in the same period.
Patient and blood sample identification
The number of detected discrepancies in ABO and RhD typing of patients due to identification or labeling errors outside the transfusion laboratory divided by the total number of patient samples tested for ABO and RhD type screenings in the same period.
Compatibility testing
The number of detected discrepancies in ABO and RhD screening of patients due to errors in the transfusion laboratory divided by the total number of ABO and RhD type screenings performed in the same period.
Traceability
The number of units for which there is no record in the hospital blood bank or blood establishment of the final destination (transfused to an identified patient, destroyed or returned to the BE) divided by the number of units issued by the HBB or BE.
4. Adverse events
Positive patient identification
Misidentification of patients is an important cause of avoidable harm in all areas of clinical practice, not only blood transfusion. Over the 12 month period February 2006 to January 2007, the UK National Patient Safety Agency received 24,382 reports of patients who were mismatched to their care in some way. Table 4.1 gives examples of adverse events caused by errors in identification and factors that may cause or predispose to errors. (http://www.jointcommission.org/SentinelEvents/SentinelEventAlert/sea_10.htm).
Reliable identification of patients depends on the use of standard operating procedures and the consistent application of strict rules for the items of data used to identify patients. Staff should be supported by systems such as the use of patient wristbands, patient identity cards, or handwritten or computer generated wristbands. Electronic systems for bedside checking of administration of blood or medicines have been successfully implemented (18067511, 18482189, 18346018). Whatever methods are used, the safety of patients depends on the acceptance and use of procedures approved by the hospital authorities. All personnel involved must understand the need for constant care and attention in adhering to the approved procedures.
Minimum essential data set for patient identification
To ensure positive identification of the patient in hospital there should be a specified set of identifying information that is agreed by the authority appropriate to the hospital. This should contain the following items:
- First name
- Last name
- Date of birth
- Sex
- Unique identifying number, such as:
- Social Security number
- National Health Service number
- Hospital identity number
Extra precautions needed to avoid misidentification
Unconscious patients
There must be a system that ensures reliable identification of patients who are unconscious or whose identity is unknown, for example after an accident. This is often done by using a Unique Emergency Number. This should be attached to the patient using a wrist band or some other locally specified method that ensures that the identity number remains attached to the patient during treatment and transfer to other departments. The blood request form and the blood sample tube must be labelled with the identical information. Once the patient’s full identity is known, the blood bank and other relevant departments should be informed.
Patients of different culture and language groups
Different cultures may have their own conventions for naming individuals leading to confusion about terms such as “family name”, “surname” and “first name”. Some individuals may not know their date of birth.
Babies in neonatal unit
Often there will be several infants in the same neonatal unit who have the same date of birth and for whom only the family name or mother’s name is available.
Table 4.1 Misidentification errors: causes and consequences
|
Misidentification Errors
|
|
|---|---|
|
|
|
| Factors that may cause or predispose to errors | Adverse events cased by errors in identification |
|
|
Table 4.2 Key points in patient information
|
Key Points
|
|---|
|
|
| Positive Patient Identification Key Messages |
|
Positive patient identification: key messages (table 4.2)
The patient must be:
- Positively identified before blood samples are taken for pre-transfusion testing
- Positively identified before blood is transfused
- Asked to confirm his or her identity:
- when a blood sample is taken
- before each unit of blood component is transfused
- Identifying information must be securely attached to the patient using a method defined in local rules
- If this identification is removed it must be quickly replaced
- The unconscious or unknown patient must be given a unique emergency number
Some hospital blood banks refuse to accept or process blood sample tubes or request forms that have incomplete or inaccurate information. This has been reported to result in a significant reduction in labelling errors. As with other critical steps, procedures for patient identification must be audited at regular intervals. Documents to assist with this type of audit are provided.
Haemovigilance
In simple language, haemovigilance means an organised system for
- observing, recording, analysing and reporting when something goes wrong
- using the lessons learned to take action to avoid it going wrong again.
Haemovigilance is an important part of the quality system for transfusion. Other methods for identifying errors, adverse events and reactions include audits of practice and the investigation of complaints. (PMID 12423521)
EU legal requirements
In the EU, certain aspects of haemovigilance are legal requirements governed by Directives which define haemovigilance as
- a set of organised surveillance procedures relating to serious adverse or unexpected events or reactions in donors or recipients, and the epidemiological follow-up of donors; 2002/98 EC (downloadable from the bottom of the page).
Clinical use of blood and blood components is not a competence for the European Union. It remains under the responsibility of the Member States. Therefore the EU legal requirements are restricted to reporting serious adverse events and reactions that are related to the quality and safety of blood or blood components,
Serious adverse reaction (SAR)
- defined by EU Directive as an unintended response in a donor or in a patient that is associated with the collection or transfusion of blood or blood components and that is fatal, life-threatening, disabling, incapacitating, or that results in or prolongs hospitalisation or morbidity. 2002/98 EC
- a serious adverse reaction must be reported if it may be due to the quality and safety of blood and blood components 2005 /61/EC (downloadable from the bottom of the page).
Serious adverse event
- defined by EU Directive as an untoward* occurrence associated with the collection, testing, processing, storage and distribution *of blood and blood components that might lead to death or life-threatening, disabling or incapacitating conditions for patients or which results in, or prolongs, hospitalisation or morbidity. 2002/98 EC (downloadable from the bottom of the page).
- a serious adverse event must be reported if it may affect the quality or safety of blood and blood components 2005/61/EC (downloadable from the bottom of the page).
For the different adverse reactions the EU Directive uses the International Society of Blood Transfusion (ISBT) definitions of transfusion reactions (http://www.ihn-org.net/Portal.aspx).
National haemovigilance systems
Serious adverse reactions and serious adverse events must be reported to the Competent Authority of each member state according to the procedures that it has specified,.
- adverse events and reactions that are due to problems in any part of the clinical transfusion process as defined in this Manual are not subject to mandatory reporting under the Blood Directive
- table 4.4 shows that adverse events and reactions may be due to the blood component itself, to errors in pretransfusion testing or administration, or to interactions between patient and transfused blood that may not reflect any error and that may not be preventable.
- each country may specify details of its haemovigilance arrangements that are additional to the requirements of the EU Directives. These may include a requirement to report events or reactions that are due to problems in the clinical transfusion process
* See Glossary
| Attachment | Size |
|---|---|
| 242 KB | |
| 62.6 KB |
4.1 Errors: causes, consequences and actions for quality improvement
Figures 1.2 to 1.7 in the Synopsis section of the site illustrate the causes and consequences of errors that can occur throughout the clinical transfusion process and give an outline of practical actions that can help to minimise risk.
4.2 Prevention and avoidance
The Netherlands’ haemovigilance scheme has estimated that up to half of all serious transfusion reactions are preventable by methods that are currently available.
Table 4.4 hows a classification of adverse transfusion reactions. This distinguishes (a) reactions that are due to an intrinsic quality defect in the blood component supplied (e.g. undetected hepatitis B infectivity) from (b) reactions that may result from a failure to select the correct product (e.g. irradiated components for patient at risk of GvHD) and (c) reactions, such as anaphylaxis or TRALI that may impossible to predict.
4.3 Some features of different national haemovigilance programmes
Established national haemovigilance programmes have developed somewhat different definitions and reporting requirements such as the examples that follow.
The Netherlands’ Haemovigilance Organization (TRIP), uses the term:
- Serious Transfusion Reaction – any incident that results in death or is life threatening to a patient, or that requires hospitalisation or prolongs hospital stay or that results in persistent significant disability. www.shotuk.org
A number of schemes including the UK scheme, SHOT use the term:
- Near miss – an error that might have harmed a patient but did not
National haemovigilance schemes do not all collect the same level of information, for example,
- the Netherlands’ scheme requires hospitals to report all incidences of transfusion of an incorrect blood component, but regards reporting of near misses as optional
- UK and Ireland concentrate on ‘serious hazards’ of transfusion, which are defined in their reporting schemes but do not accept reports of transfusion reactions that, although more common are considered to be less serious such as febrile non haemolytic reactions
- in France haemovigilance data is collected on all reactions regardless of severity www.ints.fr
These differences make it important to exercise care when comparing results among the different schemes. This is illustrated by the data from four national Haemovigilance schemes shown in Table 4.3, which shows very different rates of events, due in part to the different reporting requirements.
Table 4.4 Preventable and non preventable adverse events
| Type of adverse reaction | Related to the quality and safety of the supplied blood component? | Related to failure in clinical transfusion process? | Means of prevention |
|---|---|---|---|
|
Transfusion - transmitted bacterial infection |
Yes |
Possible due to failure to inspect component before transfusion |
Donor skin cleansing |
Transfusion-transmitted viral infection
|
Yes |
No |
Correct handling to avoid damage to containers Donor selection |
Transfusion-transmitted parasitic infection
|
Yes |
No |
Donor selection |
|
Haemolysis due to incorrect storage |
No |
Yes |
Quality assured clinical transfusion process |
|
Immunological haemolysis due to ABO incompatibility |
No |
Yes |
|
|
Immunological haemolysis due to other alloantibody |
No |
Yes |
|
|
Anaphylaxis or hypersensitivity |
No |
No |
May be unpredictable and unavoidable TRALI risk may be reduced with FFP from male donors |
|
Graft versus host disease |
No |
Yes |
Use of irradiated components for at-risk patients Use of amotosalen treated platelets |
|
Transfusion associated circulatory overload |
No |
Yes |
Avoid over-infusion. |
Figure 4.1 Types of adverse events and reactions. Serious hazards of transfusion (SHOT) UK.
Cumulative numbers of cases reviewed 1996-2008 n=5374
*New categories for 2008
Table 4.3 Adverse events and reactions: reported rates in different countries
|
International Comparison
|
|||
|---|---|---|---|
| Country | Status | Captures | Reports/1000 units |
| France (2005) | Mandatory | all | 2.8 |
| UK (2005) | Voluntary | serious | 0.20 |
| Ireland (2005) | Voluntary | serious | 1.22 |
| Netherlands (2006) | Voluntary | all | 2.9 |
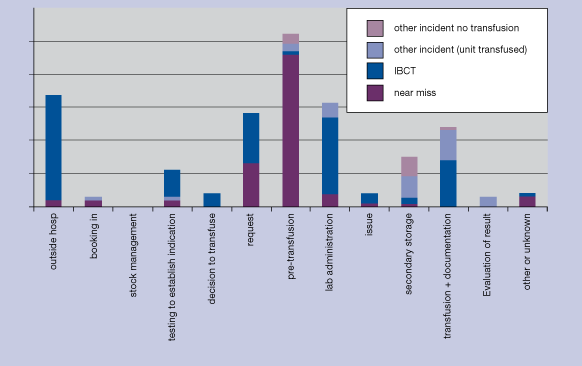
Risk management involves recording information on when errors were made, whether they were detected and how they were detected, and the reason for the error. This is sometimes called “root cause analysis”. Figures 4.2 and 4.3 show how one scheme has used its data to map the site of the first error the step in the clinical transfusion process where it occurred. In this example, the large number of incidents reported and categorised as ‘pretransfusion testing’ is mainly due to errors in collecting the pretransfusion sample rather than to errors in the blood bank laboratory. Nearly all these reports are of near misses. The corrective measure adopted in this case was to require that the blood group is always determined on two independent samples before compatible blood is issued.
Figure 4.2 Where adverse events and reactions occur in the clinical transfusion process: Netherlands haemovigilance scheme (TRIP)
Figure 4.3 Site of first error leading to potential ABO incompatibility incident Netherlands haemovigilance scheme (TRIP)
5. Documentation
This section provides guidance on documents that form an important part of the quality system. These should show how to carry out and record specific steps in the clinical transfusion process and should include guidelines on the indications for blood component transfusion. These are referred to in the manual as Standard Operating Procedures (SOPs) and Clinical Transfusion Guidelines (CTGs). Such documents are an important component of quality management. They provide guidance for the supporting processes and clinical practice of patient care and they are an essential part of the criteria against which practice can be assessed.
Because of the diversity in the ways that transfusion is organised in EU countries, this chapter is intended as a guide to what may be required but not to be prescriptive. Important areas of practice, notably the secure identification of patients, may need to be covered in several documents. It is therefore essential to ensure that information is kept consistent across documents. Documents must also be periodically reviewed and updated. This requires that there is some form of document control system. Hospital managements should ensure that as part of their quality system for transfusion, the documents listed in tables 5.1, 5.2 and 5.3 are in place and in use.
The blood establishment and hospital blood bank should have a written agreement for the provision of service, including ordering procedures, stock levels and delivery1 arrangements
Both the blood establishment and hospital blood bank should be involved in preparing and updating guidelines or procedures relating to logistics of blood components. This should include or refer to agreed procedures for the following. (tables 5.1, 5.2, 5.3)
Guidance on standard operating procedures can be found at www.eu-q-blood-sop.de
5.1 Clinical Transfusion Guidelines
Guidelines on the clinical indications for blood component transfusion should generally be available for clinical situations relevant to the hospital’s clinical activities, see the Transfusion section of this site.
Table 5.1 Hospital blood banks should have SOPs for:
| Procedure or process | Use this space to note reference to local procedure or relevant example |
|---|---|
| Stock inventory management | |
| Receipt of blood samples | |
| Pretransfusion testing | |
| Issue of blood components | |
| Emergency supply of blood components | |
| Adverse reaction/event reporting | |
| Traceability of blood components | |
| Blood components: essential information for clinicians | |
Table 5.2 Agreement between blood establishment and hospital blood bank should include
| Procedure or process | Use this space to note reference to local procedure or relevant example |
|---|---|
| Ordering blood components from the Blood Establishment | |
| Storage and transport of blood components | |
| Checking the quality of blood components at receipt | |
| Stock Management | |
| Traceability of blood components | |
| Haemovigilance | |
Table 5.3 Hospital blood banks and clinical units should have SOPs for these aspects of the clinical transfusion process:
| Procedure or process | Use this space to note reference to local procedure or relevant example |
|---|---|
| Assessing the need for blood component therapy | |
| Patient information and documenting consent from the patient | |
| Taking blood samples for pretransfusion testing | |
| Making the request for blood components | |
| Surgical Blood Ordering Schedule | |
Ordering, pretransfusion testing, issue and delivery of blood components
|
|
| Transportation of blood samples to the Hospital Blood Bank | |
| Acceptance criteria for samples received in the laboratory | |
| Thawing of FFP | |
| Transportation of blood components | |
| Pre-administration checks and bedside tests | |
| Selection and use of infusion devices (e.g. rapid infusion, neonatal transfusion) | |
| Setting up the transfusion, administering, transfusion rates | |
| Warming infusion fluids including blood | |
| Baseline observation and monitoring of the patient | |
| Management of adverse reactions | |
| Traceability of blood components | |
6. Blood components
This section provides a brief description of the main blood components. Full details of the specifications of blood components should be available from each blood establishment, which will have quality assurance procedures to maintain compliance with the approved specification. Blood establishments are regulated and inspected in accordance with the requirements of the relevant EU Directives.
Nonographs describing blood components can be found at www.edqm.eu (go to Guide to Preparation, use and quality control blood components).
6.1 Preparation of blood components
Until the late 1970s, most blood was transfused without being further processed to separate plasma or platelets. This was termed ‘whole blood’. Current practice in many EU countries is to process most or all whole blood donations into components – red cells, platelets and plasma.
In a typical blood establishment process, 450-500 ml of the donor’s blood is drawn into a plastic pack containing 63 ml of an anticoagulant-preservative solution such as Citrate Phosphate Dextrose (CPD) or CPD–Adenine. The citrate binds calcium and acts as an anticoagulant, and the glucose and adenine support red cell metabolism during storage. The whole blood unit may be filtered to remove white cells, most of the plasma is removed, and an additive solution, formulated to support erythrocyte metabolism, is added to the remaining red cells.
Platelet concentrate may be prepared either from the white cell and platelet layer (the so-called buffy coat) or from platelet rich plasma. Red cells, platelets, plasma and white cells can also be collected by apheresis.
Directive 2002/98 EC lists names and specifications of red cell, platelet and plasma components. These are summarised in table 6.1 at the end of this chapter. This section of the manual provides information about some of these components that are in common use.
In the manual, the term ‘red cell unit’ is used to denote the red cells from one standard blood donation.
6.2 Blood component label
The blood component label should comply with the relevant national legislation and international agreements. Most EU countries use the international labelling system known as ISBT 128 www.iccbba.org (R5 downloadable from end of page).
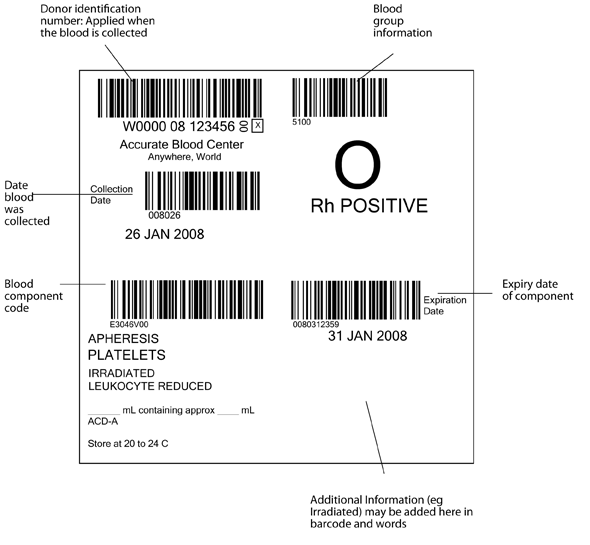
The pack label contains essential information about the blood component, as illustrated in Figure 6.1 and 6.2. The ISBT system requires that the following information be shown in barcode and eye readable form, in the four quadrants of the label.
- Top left: the unique donation number, containing a 5 digit code for the blood establishment, two digits for the year of collection, and a six digit donation number. The blood establishment name and date of the collection must be in eye readable form, (and in figure 6.1 are also shown as a barcode)
- Top right: ABO and RhD blood group
- Lower left: The identification code for the type of blood component (eg red cells, leucocyte depleted in additive solution)
- Lower right: The expiry date of the component. Additional information (eg irradiated) may be added in this quadrant in eye readable and barcode form (see fig 6.2)
Detailed information about barcoding of blood components can be found at www.iccbba.org
Figure 6.1 International ISBT 128 blood component label as specified in the ICCBBA Standard. www.iccbba.org
Directive 2002/98/EC requires that the following information should be shown on the label:
- Official name of the component
- Volume or weight or number of cells in the component (as appropiate)
- Unique numeric or alphanumeric donatior identification
- Name of producing blood establishment
- ABO Group (not required for plasma intended only for fractionation)
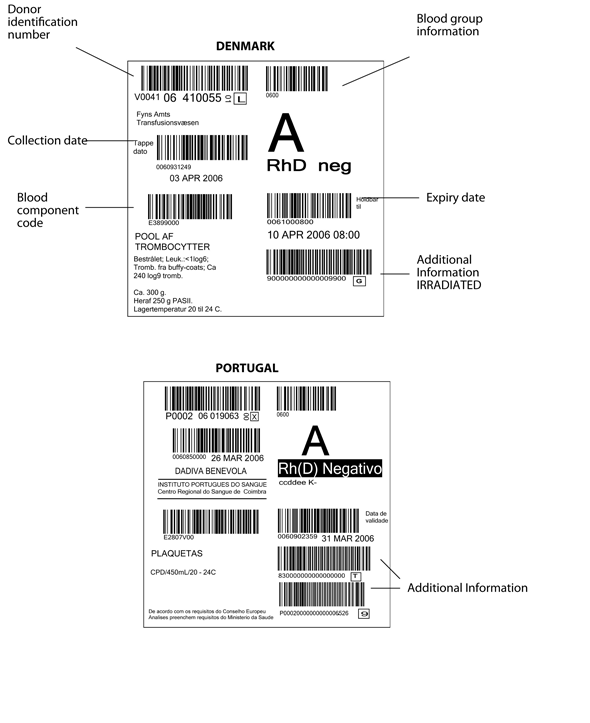
Figure 6.2 Blood component labels from EU countries
Above: Denmark, Below: Portugal
| Attachment | Size |
|---|---|
| 829.2 KB |
6.3 Labelling of blood prepared for an individual patient
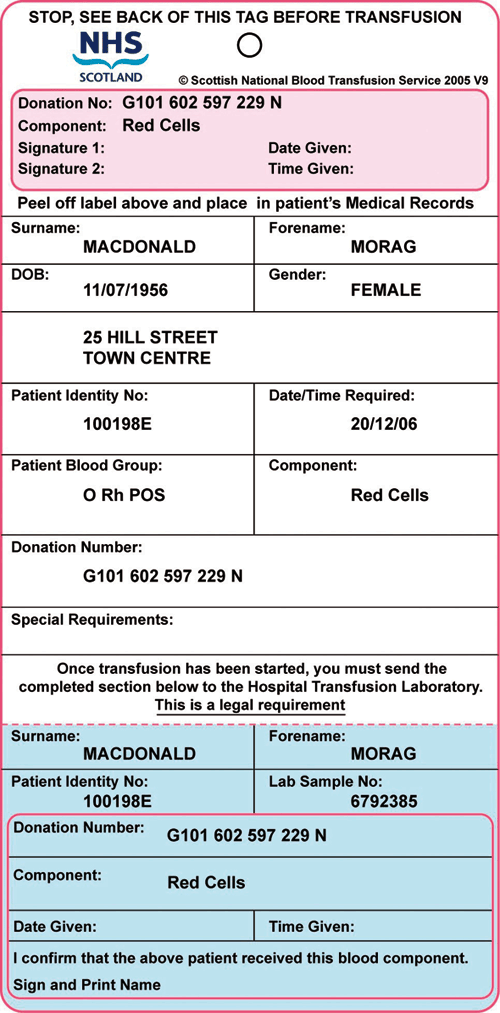
Components issued for an individual patient should also have a label that identifies the patient for whom the blood component has been prepared. This is often referred to as the Compatibility Label. It must be firmly attached to the pack and may be an adhesive label or a tie-on tag. Figure 6.3 shows an example of such a label that has been designed to provide documentary evidence of traceability.
Figure 6.3
Example of a compatibility label. It must be firmly attached to the pack and may be an adhesive label or a tie-on tag. This example can be used to provide documentary evidence of traceability.
6.4 Outline of blood component preparation and composition
This section gives a general overview of the information that clinicians may need about blood components. Other examples are given at www.edqm.eu (Search for Blood Transfusion) Guide to preparation, use and quality control of blood components) and national guides (attached at bottom of this page).
Red Cell Components
Whole blood
Typically this contains 450-500 ml of donor blood that has been collected into a pack containing 63 ml of an anticoagulant solution such as CPD.
Red cells in additive solution
Typically all but 20 ml of plasma is removed from the collected whole blood and replaced with an additive solution designed to optimise red cell preservation, such as saline solution containing added adenine, glucose and mannitol (also called SAGM, SAGMAN, Adsol or optimal additive solution). It should contain at least 45 g of haemoglobin per unit. The EU Directive refers to this as “red cells in additive solution”. Other variants of red cell components include red cells that are leucocyte-depleted, have buffy coat removed, or are collected by apheresis.
| Attachment | Size |
|---|---|
| 858.66 KB | |
| 628 KB | |
| 1.74 MB | |
| 1.55 MB |
6.5 Platelet components
Often referred to as “platelet concentrate”.
Recovered or apheresis
Platelets can be prepared by centrifuging a whole blood donation (often called recovered platelets) or collected by apheresis. Platelets prepared by each method have similar efficacy, but use of apheresis platelets exposes the recipient to the blood of fewer donors. The yield of platelets recovered from four to six whole blood donations should be 300x109 to 350x109 platelets in about 300 ml of plasma (the plasma is required to maintain platelet function during storage).
A single apheresis donation of platelets has comparable content of platelets and plasma. The use of a platelet additive solution allows platelets to be stored in reduced amounts of plasma. Platelet function is best maintained by storage at 22°C with agitation. As this temperature favours growth of some bacteria, some centres culture platelet concentrates prior to release from storage with the aim of reducing the risk of bacterial contamination. Platelets are generally stored for up to five days and some countries permit storage for seven days with special precautions.
6.6 Plasma components
Fresh frozen plasma (FFP) is separated and frozen, usually within six to eight hours after collection, to preserve factor VIII content. Other plasma components are:
- Cryoprecipitate – this is prepared by controlled thawing of frozen plasma to precipitate high molecular weight proteins, including factor VIIIc, von Willebrand factor and fibrinogen
- Cryoprecipitate depleted plasma – this is FFP from which cryoprecipitate has been prepared, leading to reduced concentration of fibrinogen and factor VIII
Leucocyte depletion
Removal of leucocytes to a level of less than one million per component by filtration or during collection of blood components by apheresis is normal practice in a number of EU countries.
Advantages of leucodepletion include a marked reduction in alloimmunisation to HLA antigens and in the risk of infection by intracellular viruses such as cytomegalovirus. Leucodepletion of red cells may also be associated with improved outcomes in some groups of patients.
6.7 Pathogen-reduced blood components
Processes that reduce or abolish the infectivity of microrganisms in blood components offer an additional level of security against transfusion transmissible infections, including those for which screening tests are not currently available. (19304113).
Plasma
Several processes are available that have been shown to cause substantial reductions in infectivity while causing only moderate reduction in activity of fibrinogen and other plasma proteins. These processes utilise methylene blue, amotosalen, or riboflavin (single donor units), or a solvent detergent treatment (applied to pool of multiple units). An alternative approach is the use of quarantined plasma (PMID 18583192).
Platelets
Platelets pose a risk of bacterial contamination because they are stored at 22°C. Bacterial culture of platelets during the storage period is used by some organisations to minimise this risk. A process for pathogen inactivation of platelets is now CE marked and in use in several countries. A further large clinical trial of efficacy and safety is due to report its findings.
Red cells
Processes for pathogen reduction of red cell components have not completed clinical trials.
6.8 Cytomegalovirus (CMV)
Cellular blood components may result in the transmission of CMV to groups of patients at risk. Practice in many EU countries is to use leucodepleted blood components to avoid this risk. In some countries the use of blood components that test negative for CMV antibody is recommended for patients at special risk of CMV infection (PMID 11149975).
6.9 Transfusion Associate Graft versus Host Disease (TA GvHD)
Transfusion can cause Graft Versus Host Disease. TA GvHD causes tissue and organ damage that is usually fatal. There is an intensive immunologic reaction mediated by transfused immunocompetent lymphocytes directed against an immunocompromised recipient, or one who shares an HLA haplotype with the donor.
The risk of TA-GVHD can be avoided by irradiation of cellular blood components or by treating platelet components with amotosalen. This inactivates T lymphocytes remaining in the component so that they are unable to engraft (PMID 18544121, 17655584, 15744235)
Irradiation may employ Gamma irradiation using a Cs137 or Co59 source, or special X-ray equipment that is now available for this purpose.
6.10 Use of washed red cells
When a patient has experienced severe allergic reactions associated with transfusion, reactions to subsequent transfusions may be prevented by the use of red cells that have been washed in sterile saline using special equipment.
This should remove residual plasma proteins cytokines or antibodies that may be the cause of the reactions. Saline washed RBCs must be used within 24 hours after washing since the saline contains no red cell nutrients and the original collection bag has been entered with consequent risk of bacterial contamination.
6.11 Clinical indications for transfusion of blood components
Summary information about the indications for use of blood components is provided in the Transfusion section of the site.
6.12 Component specifications from Directive 2004/33/EC
These are summarised in table 6.1
Table 6.1 Summary of specifcations for blood components Directive 2004/33/EC.
This table contains the information given in annex v, para 2.4
| Blood Component | Haemoglobin | Haemolysis | Leucocyte Content | |
|---|---|---|---|---|
|
RED CELLS: Volume |
||||
|
Red Cells |
Not less than 45 g per unit |
Haemolysis: Less than 0.8% of red cell mass at the end of the shelf life |
||
|
Red Cells, buffy coat removed |
Not less than 43 g per unit |
|||
|
Red Cells, leucocytedepleted |
Not less than 40 g per unit |
< 1 × 106 per unit |
||
|
Red Cells, in additive solution |
Not less than 45 g per unit |
|||
|
Red Cells, buffy coatremoved, in additive solution |
Not less than 43 g per unit |
|||
| Red Cells, leucocyte-depleted, in additive solution | Not less than 40 g per unit |
<1 × 106 per unit |
||
|
Red Cells, apheresis |
Not less than 40 g per unit |
|||
|
Whole blood Not referenced in annex V, para 2.4 of Directive 202/98 EC |
||||
|
PLATELETS: Volume Valid for storage characteristics to maintain product within specifcations for pH |
Platelet content |
pH |
Leucocyte content |
|
|
Platelets, apheresis |
Variations permitted within limits that comply with validated preparation and preservation conditions |
6,4 – 7,4 corrected for 22 °C, at the end of the shelf life |
||
|
Platelets, apheresis, leucocyte-depleted |
< 1 × 106 per unit |
|||
|
Platelets, recovered, pooled platelet-rich plasma method |
< 0,2 × 109 per single unit |
|||
|
Platelets, recovered, pooled buffy coat method |
< 0,05 × 109 per single |
|||
|
Platelets, recovered, pooled, leucocyte depleted |
< 1 × 106 per pool |
|||
|
Platelets, recovered, single unit |
< 0,2 × 109 per single unit (platelet-rich plasma method) |
|||
|
Platelets, recovered, single unit, leucocyte depleted |
< 1 × 106 per unit |
|||
|
PLASMA |
Factor VIIIc |
Fibrinogen |
Total protein |
Residual cellular content |
|
Plasma, fresh-frozen |
= / > 70 % value of the freshly collected plasma unit |
Not less than 50 g/l |
Red cells: < 6,0 × 109 /l Leucocytes: < 0, 1 × 109 /l Platelets: less than 50 × 109 /l |
|
|
Red cells: < 6,0 × 109 |
||||
|
Cryoprecipitate |
= / > 70 international units per unit |
= / > 70 140 mg per unit |
||
|
GRANULOCYTES |
Granulocyte content |
|||
|
Granulocytes, apheresis |
>1 × 1010 granulocytes per unit |
|||
7. Clinical
Optimal use of blood is defined in this manual as ‘The safe, clinically effective and effcient use of donated human blood.” However, for many of the familiar and widely accepted indications for transfusion it is a fact that there is surprisingly little high quality evidence to establish the effectiveness of transfusion therapy. As a result, clinical transfusion guidelines must often be based on inadequate information. Information in this chapter about the quality and grading of evidence for clinical practice guidelines has been drawn from the German Guidelines for Therapy with Blood Components and Plasma Derivatives (2009). Another useful sources is the database of systematic reviews at the website www.transfusionguidelines.org.uk (go to Evidence Library).
7.1 Epidemiology of blood use
The use of blood components per capita, varies widely, even among the populations of countries that have similar levels of health care. This is despite the existence of broadly similar clinic transfusion guidelines in most EU countries. This wide variation may in part be a result of differences in the demographics or patterns of disease in different populations (PMID 7889138, 19210324, 8023386). However a number of studies have shown that, at least for surgical transfusion, much of the variation cannot be explained by these factors. The low blood requirements of some surgical teams may reflect their attention to the many details of patient management that influence the need to transfuse, including the appropriate use of lower haemoglobin thresholds for transfusion, surgical and anaesthetic techniques, avoidance of hypothermia and the use of “blood sparing” technologies.
7.2 Which patients get transfused?
Studies in several European countries show that although patients undergoing surgery and treatment for malignant disease are major users of transfusion, a substantial proportion of all transfusions are used for patients who do not belong to any simple category, who are in older age groups and who have essentially “medical” conditions, often with multiple diagnoses, interventions and episodes of hospital care (PMID 19500320).
7.3 To transfuse or not?
The challenge of making an acute clinical decision about transfusion is to assess the likely benefts for the individual patient (PMID 12076437). One way to aid clinical decision-making is to use a simple checklist (downloadable from end of page>) such as the following to help focus the decision):
- What improvement in the patient’s clinical condition am I aiming to achieve?
- Can it be achieved without transfusing?
- Can I minimize blood loss to avoid the need for transfusion?
- Are there any other treatments I should give before making the decision to transfuse (such as intravenous replacement fuids oxygen, inotropes)?
- What are the specifc clinical or laboratory indications for transfusion for this patient at this time?
- What are the risks of infection or some other severe adverse event?
- Do the benefts of transfusion outweigh the risks for this particular patient?
- Will a trained person respond immediately if an acute transfusion reaction occurs?
- If this blood was for my child, or myself would I accept the transfusion or not?
- Have I recorded on the patient’s chart (and signed) my decision and my reasons for transfusion?
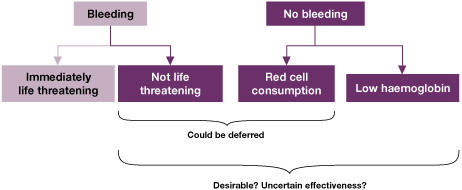
Decision-making can be relatively straightforward when a patient has a life-threatening major haemorrhage, bleeding associated with profound thrombocytopenia, or severe, disabling symptoms of anaemia associated with cancer chemotherapy. Indications for transfusion may also be clear in conditions such as thallasaemia or myelodysplastic disease. The decision can be much less clear – for example in an elderly patient, who has a haemoglobin concentration of 80g/l, has no evident symptoms of anaemia, is haemodynamically stable and is not bleeding.
Figure 7.2 What makes us transfuse red cells
| Attachment | Size |
|---|---|
| 51.08 KB |
7.4 Urgent and emergency transfusion – major bleeding
A single patient with catastrophic bleeding can be a major challenge for the clinical and blood bank teams. When blood is required very rapidly it is extremely important to have clear communications between clinicians and the blood bank. Clinical and blood bank experience indicates that delays in providing blood in a life-threatening emergency can occur for various reasons and contribute to mortality in critical situations such as obstetric haemorrhage. www.cmace.org.uk
Hospitals should have a Major Haemorrhage Procedure that identifes roles, responsibilities and communication routes. (Example downloadable from end of page)
There should also be a clinical transfusion guideline for management of major bleeding. (example: PMID 17107347, 19135193)
Rehearsals (‘fre practices’) to familarise medical, nursing, laboratory and transport staff and to test the procedure.
Following road traffc accidents and other disasters several unconscious injured patients may arrive at the hospital within a short period, creating risks due to problems in identifying the patients. These are situations when it is vital for all the team to know and use the Major Haemorrhage Procedure (table 7.1).
Table 7.1 Example of a Major Haemorrhage Procedure
There should also be a clinical transfusion guideline for management of major bleeding.
|
Major Haemorrhage Procedure
|
||||
|---|---|---|---|---|
| Example of a Major Haemorrhage Procedure | ||||
|
||||
|
||||
|
||||
|
||||
|
||||
|
||||
|
||||
|
||||
|
||||
| Attachment | Size |
|---|---|
| 44.48 KB |
7.5 Clinical conditions that require a clinical transfusion guideline
Examples are given of procedures where possible and thses are currently used in hospitals in countries participating in the project.
Table 7.2 Clinical situations for which there should be transfusion guidelines
|
Major Haemorrhage Procedure
|
|
|---|---|
| Situation | Use this space to note reference to local clinical transfusion guideline or relevant example |
|
Blood ordering and supply in major haemorrhage |
|
|
The management of major haemorrhage in |
|
|
|
|
|
|
|
|
Critical Illness (transfusion in the intensive therapy unit) |
|
|
Preoperative assessment and optimisation |
|
|
Predeposit autologus blood – collection and transfusion |
|
|
Management of preoperative patients on drugs that affect haemostasis, such as
warfarin, heparin, clopidogrel
|
|
|
Peri-operative blood management and blood saving techniques/drugs |
|
|
Inherited coagulation disorders |
|
|
Acquired coagulation disorders |
|
|
Disseminated intravascular coagulation |
|
|
Thrombocytopenia and thrombocytopathy, TTP |
|
|
Prenatal and neonatal transfusion |
|
|
Haemolytic disease of the newborn: prevention and management |
|
|
Neonatal: exchange, intrauterine and top-up transfusion |
|
|
Chronic anaemia due to haematological disorders |
|
|
Myelodysplasia |
|
|
Haemoglobinopathies |
|
|
Autoimmune haemolytic anaemia |
|
|
Malignant haematological disorders: bone marrow failure |
|
|
Transplantation of haemopoietic stem cells |
|
|
Management of patients refusing blood transfusion |
|
7.6 Evidence: Systematic reviews and clinical guidelines
Systematic Review
This is a a review of the literature on a topic that
- is based on comprehensive searching of all relevant sources
- uses explicit criteria to assess the eligibility and methodological quality of the studies.
- uses established methods to assess the eligibility and methodological quality of the results
- may involve bringing together the of the results of several comparable studies to increase the strength of the conclusions that can be drawn (sometimes called meta analysis)
Systematic reviews relevant to transfusion can be found at www.transfusionguidelines.org.uk.
The Cochrane Library at http://www3.interscience.wiley.com is a comprehensive source of clinical trial reports and systematic reviews.
Clinical Guideline
Many important aspects of transfusion practice do not have a firm basis of evidence from well conducted randomised controlled clinical trials that have the capability to identify the most effective process or treatment. As a result, clinical guidelines often must be based on the best available information such as observational studies, case reports and properly developed concensus of professional opinion.
Example
The full document can be found at www.arzt.de (English transation downloadable from the bottom of the page). These guidelines were developed over several years on the basis of reviews of the current literature and show.
- How the quality (level) of the evidence was graded
- How the recommendations for practice were constructed.
| Attachment | Size |
|---|---|
| 869.92 KB |
7.7 Evidence-based recommendations for practice
The following is an extract from the 2009 guidelines of the Bundesaerzekammer (German Medical Association) www.arzt.de
Grading of recommendations
- Level 1: based on available data, the benefits to the patient of complying with the recommendation are judged by experts to outweigh the potential risk
- Level 2: if there are no definite data on the risk-benefit ratio
Grading of level of evidence
- Level A: data from large, prospective, randomised studies
- Level B: data from several prospective studies with conflicting results or with methodological flaws
- Level C: data from case reports and non-randomised studies.
- Level C+: data from case reports and non-randomised studies are unambiguous and confirmed by several investigations
Consequences of the recommendations
Both the level of evidence based on underlying data and the level of recommendation, reflecting the risk-benefit ratio impact on recommendation for medical practice (table 7.3).
Table 7.3 Classification of recommendations for clinical transfusion guidelines
Reproduced from: cross sectional guidelines for therapy with blood components and plasma derivatives, 4th revised edition 2009. Bundesaertztekammer (German Medical Association)
| Level of recommendation | Risk-benefit ratio | Level of evidence | Assessment of the methodological validity of the underlying data | Overall assessment, classification | Implications | Key words |
|---|---|---|---|---|---|---|
| 1 | Unambiguous | A | Randomised, controlled studies without essential methodological flaws with unambiguous results | 1 A | Strong recommendation. Valid for most patients |
shall |
| 1 | Unambiguous | C+ | No randomised, controlled studies, but unambiguous data available | 1 C+ | ||
| 1 | Unambiguous | B | Randomised, controlled study with methodological flaws. Despite unambiguous results of the study, it cannot be safely ruled out that methodical flaws have influenced the results | 1 B | Strong recommendation. Probably valid for most patients |
|
| 1 | Unambiguous | C | Observational studies without control group, but with convincing results | 1 C | Medium-strong recommendation, seems to be plausible, may be changed once improved data becomes available |
Should |
| 2 | Ambiguous | A | Randomised, controlled study without methodological reservations, but with conflicting results | 2 A | Medium-strong recommendation, depending on the individual case, a different course of action may be indicated. The interpretation of results by the Working Group Guidelines are taken into account in the recommendation |
|
| 2 | Ambiguous | C+ | No randomised, controlled studies, but data can be extrapolated from other studies | 2 C+ | Weak recommendation, depending on the individual case, a different course of action may be indicated. The interpretation of results by the Working Group Guidelines are taken into account in the recommendation |
Can |
| 2 | Ambiguous | B | Randomised, controlled study with severe flaws | 2 B | Weak recommendation, depending on the individual case, a different course of action may be indicated |
Can |
| 2 | Ambiguous | C | Observational studies, case reports | 2 C | Very Weak recommendation, depending on the individual case, a different course of action may be indicated |
Could |
| Cross sectional guidelines for therapy with blood components and plasma derivatives, 4th revised edition 2009. Reproduced with permission from Bundesaertsekammer (German Medical Association). | ||||||
7.8 Key points about the clinical indications for transfusing blood components
Red Cells
Major haemorrhage
For patients who are shocked and anaemic red cell transfusion to increase the circulating red cell mass can relieve clinical features that are caused by insufficient oxygen delivery (PMID 14556774, 9504576). Circulating blood volume must be corrected with other fluids (PMID 15163774, 12535407, 11279761). Mortality rates are high in patients who do not receive blood.
Acute anaemia
A randomised trial in ICU patients suggested that transfusion of red cells to achieve a higher haemoglobin concentration target appears to offer no benefit over more conservative transfusion to achieve a lower target Hb concentration. The exception to this may be patients with cardiovascular disease. Table 7.4 shows a recent evidence-based national clinical transfusion guideline for transfusion of red cells in acute anaemia
Germany Haemotherapy Guidelines downloadable from the bottom of the page.
Table 7.4 Evidence based national clincal transfusion guideline for transfusion of red cells in acute anaemia
Reproduced from: cross sectional guidelines for therapy with blood components and plasma derivatives, 4th revised edition 2009. Bundesaertztekammer (German Medical Association)
| The decision on transfusion for each patient should take account of that patient’s haemoglobin (Hb) concentration, capacity to compensate for acute anaemia, and risk factors | |||
|---|---|---|---|
| Hb concentration alone is not an adequate measurement of oxygen supply. If the patient is hypovolaemic the Hb concentration does not correctly reflect the red cell mass in an individual patient and it may be necessary to deviate from the recommendations below | |||
| range of haemoglobin concentration | capacity to compensate: risk factors | recommendation on red cell transfusion | strength of recommendation * |
| < 6 g/dl (3.7mmol/l) | Yes | 1C+ | |
| > 6-8 g/dl (3.7-5 mmol/l | adequate compensation: no risk factors | No | 1C+ |
| limited compensation: risk factors such as coronary artery disease, cardiac insufficiency, cerebrovascular insufficiency | Yes | 1C+ | |
| symptoms of anaemic hypoxia or decompensation (physiologic transfusion trigger) eg tachycardia, hypotension, ECG ischemia, lactic acidosis | Yes | 1C+ | |
| > 8-10 g/dl (5.0-6.2 mmol/l | symptoms of anaemic hypoxia or decompensation (physiologic transfusion trigger) e.g. tachycardia, hypotension, ECG ischemia, lactic acidosis | Yes | 2C |
| > 10g/dl (6.2 mmol/l) | No | 1A | |
Neonatal ICU patients
Transfusion of red cells to achieve a higher haemoglobin concentration target in patients who require transfusion appears to offer no benefit over more conservative transfusion to achieve a lower target Hb concentration (PMID 19117884). Target haemoglobin levels used in the key randomised controlled clinical trial depended on the age and condition of the infant.
Thalassaemia major
In countries where thalassaemia is still prevalent, it can account for a large proportion of the clinical requirement for red cell transfusion. In many countries, as a result of successful prevention programmes, most cases are now in older individuals. Red cell transfusions are typically given at two to four weekly intervals to maintain a mean Hb around 12g/dl. The aim is to fully relieve the symptoms of anaemia and suppress the patient’s own increased abnormal red cell production in the marrow (ineffective erythropoiesis). This is the cause of the skeletal abnormalities and spleen enlargement seen in under-treated patients. All patients need iron chelation therapy to prevent progressive and ultimately fatal organ damage (PMID 18413891).
Symptomatic anaemia patients with haematological malignancies or solid tumours:
The local clinical management protocol should define the range within which a patient’s haemoglobin should be maintained. A suggested arbitrary guide is to maintain Hb at not less than 9.0g/dl. As a result of complications associated with the use of erythropoetin in patients with cancer, guidelines in a number of countries now discourage or restrict its use in this situation (PMID 16705125).
| Attachment | Size |
|---|---|
| 869.92 KB |
7.9 Platelets
The normal range for the platelet count in peripheral blood at all ages is 150-400 x 109/l. A platelet count below this level does not in itself indicate a need for platelet transfusion. Isolated thrombocytopenia, in the absence of any other abnormality, is unlikely to be complicated by serious spontaneous haemorrhage if the count remains above 5 -10 x 109/l. Recent studies indicate that the clinically stable patient is unlikely to benefit from prophylactic platelet transfusion if the count is greater than 10 x 109/l. A higher threshold for transfusion is generally advised in the presence of sepsis. However, some experts question the usefulness of the platelet count in the peripheral blood as a guide to the risk of bleeding or as a means for assessing the effect of platelet transfusion.
Clinical transfusion guidelines for platelet transfusion usually cover the management of bleeding during surgery or patients with bone marrow suppression and the prevention of bleeding in patients with low platelet count due to bone marrow suppression or other causes (PMID 19109560, 15495093, 15584985, 16351634). Some guidelines specify target platelet counts. In clinical practice the recommended target platelet counts may not be achieved even with large doses of platelets.
The following is an extract from the 2009 Guidelines of the German Medical Association
Major haemorrhage:
- Transfuse if count <50 × 109/l, or
- In multiple or CNS trauma < 100 × 109/l (recommendation level 2C)
Thrombocytopenia due to chemotherapy
- Transfuse if count <10 × 109/l if not bleeding and no other risk factors (recommendation level 1A)
- Transfuse if count <20 × 109/l if at risk due to sepsis, antibiotics, abnormal clotting (recommendation level 2C)
- Transfuse if there is evident bleeding (recommendation level 1C)
Invasive surgical procedures
- Transfuse if count <50 × 109/l: < 70-100x109 in procedures, such as neurosurgery, where bleeding carries higher risks (recommendation level 1C)
Invasive diagnostic interventions
- Guidance depends on individual procedure, patient risk factors for bleeding, and risk to patient if bleeding occurs
7.10 Fresh Frozen Plasma
Although FFP is widely used, there are few well-founded indications. A systematic review of all randomised trials of FFP indicates that most clinical indications for FFP that are often recommended by transfusion practice guidelines are not supported by evidence from randomised trials.
Typical clinical guideline for plasma transfusion
Major haemorrhage
Coagulopathy with a prothrombin time prolonged > 50% is likely after replacement of 1-1.5 blood volumes. Initial dose of FFP 15-20 ml/kg. Further doses only if bleeding continues and guided by PT and APTT (1C)
Thrombotic thrombocytopenic purpura (TTP)
Plasma exchange with FFP is effective in many cases (recommendation level 1A).
Other indications
Replacement of coagulation factor deficiency, if the appropiate plasma derivative or recombinant product is not available.
7.11 Fibrinogen replacement
In many EU countries, a fibrinogen product made by plasma fractionation is used for fibrinogen replacement in dysfibrinogenaemia and acquired hypofibrinogenaemia seen in
massive transfusion and DIC. An alternative is cryoprecipitate.
7.12 Frequently asked questions about blood components
Fresh or stored red cells for transfusion?
A much cited study suggested that transfusion of stored red cells could actually impair regional oxygenation but a recent blinded, randomised, controlled study comparing the effect of fresh versus stored leucocyte-depleted red cells on systemic and regional oxygenation in ICU patients showed no definitive evidence that fresh red cells have better oxygen delivery in critically ill patients (PMID 14758149) (See download at bottom of page). A study of the effect of acute anaemia on cognitive function in healthy subjects detected no difference in the response when haemoglobin concentrations were restored with fresh or stored autologous red cells (PMID 16645441). The TRICC clinical trial (PMID 9971864) suggested that some ICU patients maintained at a lower Hb concentration, and so receiving less transfusion, may have improved outcomes. One interpretation was that this could be associated with some adverse effect of transfusing stored red cells. Randomized trials are in hand to investigate this hypothesis. Large observational studies in cardiac surgery have also suggested poorer outcomes with longer stored red cells. At present, it remains to be conclusively shown in prospective studies whether the use of fresh red cells offers benefits for critically ill patients (PMID 12393351).
Is there a case for single unit transfusion of red cells?
It is often stated that there is no case for giving a single unit transfusion, but in some cases, a single unit may be an appropriate dose. For example in a 40 kg patient with hypoxic signs or symptoms attributed to a Hb concentration of 7g/dl, a single unit of red cells may be quite sufficient to relieve symptoms (and to raise the Hb concentration by 1-2g/dl). Use of a second unit in such a case exposes the patient to additional and unnecessary risks.
Whole blood vs a red cell component?
The concept of blood component therapy (together with the requirement for plasma for fractionation) has encouraged the widespread use of red cell concentrates in most developed countries, although in some other areas of the world, most transfusions are given as red cells (R67). The clinical experience of military surgical teams is that the early administration of plasma with red cells (in approximately equal volumes) appears to be associated with better achievement of haemostasis. Whole blood may be appropriate for a patient with acute bleeding who requires both red cells and expansion of plasma volume. In cases when disseminated intravascular coagulation (DIC) contributes to the blood loss, it may be logical to use whole blood (or leucocytedepleted whole blood) since it contains at least a part of the total dose of fibrinogen and stable clotting factors that the patient requires and could reduce the need for plasma units from other donors.
Is fresh frozen plasma safe?
Worldwide, the largest avoidable risk to patients from transfusion is probably due to the transfusion of fresh frozen plasma (FFP) for unproven clinical indications. Plasma is just as likely as whole blood to transmit viral infections (other than those that are strictly cell associated). In any area where blood safety testing may be unreliable, transfusion of FFP, unless it is pathogen reduced, can be an important source of transmission of these infections.
Is fresh frozen plasma clinically effective?
There is a poor level of evidence to support many of the traditional indications for transfusing FFP (PMID 15198745). This is reflected in the recent clinical guidelines e.g. from Germany and UK. FFP should be used only to replace rare clotting factor deficiencies for which no virus-safe fractionated plasma product is available or when there is a multifactor deficiency due to severe bleeding and DIC. Other indications for FFP are the management of thrombotic thrombocytopenic purpura (TTP) and haemolytic uraemic syndrome (HUS), in which plasma infusion or plasma exchange with FFP is effective (PMID 17266701).
Does Fresh Frozen Plasma have to be used immediately after thawing?
After thawing, the level of factor VIII falls rapidly. Factor V also falls, more slowly, but level of fibrinogen and the other haemostatic proteins is maintained. Guidelines in some countries permit the use of plasma that has been stored in the blood bank for up to 24 hours after thawing. This has the advantage that plasma can be released quickly when required for urgent management of massive bleeding. In some countries, liquid plasma (never frozen) is used.
| Attachment | Size |
|---|---|
| 1.55 MB |
7.13 Avoiding the need to transfuse: planned surgery
Table 7.5 Framework for managing the preoperative patient to minimise the need for allogeneic red cell transfusion
For background information try www.nataonline.com
| Time period | Manage haemoglobin level | Manage haemostasis | Blood salvage and transfusion |
|---|---|---|---|
| Preoperative Preadmission clinic | Assess for anaemia: diagnose and treat with haematinics and epoetin if indicated | Detect and manage haemostatic defects. Stop anti-coagulants and anti-platelet drugs if safe to do so. | Arrange for intraoperative blood salvage to be available if it is appropriate for the planned operation. |
| During surgery Surgical and anaesthetic techniques | Monitor haemoglobin, haematocrit or blood loss as a guide to red cell replacement | Keep the patient warm, as cold impairs blood clotting. Rapid haemostasis testing to guide blood component replacement. Consider use of tranexamic acid where large blood loss is expected. | Use intraoperative blood salvage |
| Post-operative Control Hb concentration, manage blood loss | SOP for post-op check of Hb when haemoglobin should be checked. Minimise blood taken for laboratory samples | SOP specifying blood transfusion thresholds and targets. SOP to trigger surgical re-exploration at specified level of blood loss. Post-operative blood salvage | |
Table 7.5 provides a simple framework managing the patient waiting planned surgery so as to minimise the need for perioperative transfusion.
The following techniques have all been developed as means of reducing transfusion requirements. While some have been shown to achieve this result there is relatively little knowledge about potential risks. A recent randomised clinical trial comparing three antifibrinolytic agents has demonstrated the importance of obtaining such evidence. (See Aprotinin, below)
Preoperative autologous blood deposit (PABD)
The patient donates one or more units of his own blood which is stored till the time of surgery. May be useful for patients for whom it is very difficult to obtain compatible red cells. May reduce use of allogeneic red cells but does not reduce total red cell use when reinfused units are taken into account.
Acute normovolaemic haemodilution (ANH)
Blood is collected from the patient immediately before surgery and reinfused during or after the procedure. Evidence indicates that the procedure does not reduce transfusion requirements.
Intraoperative blood salvage
Blood lost during surgery is collected, washed to remove plasma and debris, and reinfused.
Postoperative salvage
Blood from wound drains is reinfused with or without washing.
Inhibitors of fibrinolysis
Those currently available are tranexamic acid and in some countries epsilon-aminocaproic acid. Aprotinin, the antifibrinolytic that had been extensively used for many years has recently been withdrawn because in a large randomised trial there was excess mortality in patients receiving this drug compared with those receiving tranexamic acid or EACA (PMID 18480196, 19050037).
Erythropoietin (EPO, epoietin)
EPO is a potent stimulator of red cell production. The drug is made by genetically engineered expression of the human erythropoetin gene. It is highly effective in the anaemia of chronic renal failure. Studies in patients with malignant disease have shown an increase in cancer recurrence and mortality. The risk of hypertension and thrombosis increases if the dose raises the patient’s Hb concentration to near normal levels. Parenteral iron preparations are often used with EPO to deliver the iron required for rapid erythropoiesis (PMID 16999756).
Do these technologies reduce the need for donor blood transfusion?
Clinical trials to answer this question have been subject to systematic reviews with meta-analysis. These methods reduce the use of allogeneic transfusion but may have other consequences. For example, predeposit autologous transfusion usually increases the total amount of red cell units transfused when both autologous and allogeneic units are counted.
7.14 Informing patients
In EU member states where data are available the risks associated with receiving a transfusion are small in the context of the totality of risks of hospital care. However, as part of an effective quality system, patients who are able to communicate must be informed in good time about their treatment. Formal consent for transfusion is a requirement in some countries. Regardless of any legal requirement, the clinician has a professional duty to make sure the patient knows if and why a transfusion is required. The discussion should include the reasons why transfusion may be needed and the risks and benefits of receiving blood, (and in some circumstances of not receiving it). There are links to examples of information prepared for patients on the website.
The pre-admission clinic for elective surgery is an ideal opportunity to provide information about transfusion as part of the information given to the patient about the whole process of care. Many EU countries have information leaflets available for patients. Clinical notes should record that the patient has been given information about transfusion.
Questions frequently asked by patients
Figure 7.3 provides some information that may help in responding to questions that patients’ ask about transfusion.
Figure 7.3 Answering patients’ questions about transfusion
(for another example see http://sunnybrook.nextmovelearning.com)
8. Blood bank
Role of the hospital blood bank
Quality in transfusion practice must apply to the hospital blood bank or equivalent, because it plays a vital role in ensuring that the correct blood component is supplied for the patient.
The laboratory aspect of the transfusion process is carried out in different ways across the countries of the EU. In some settings a local hospital blood bank manages the blood component inventory and the clinical blood transfusion laboratory services. Elsewhere, the blood establishment provides compatible blood directly to hospitals.
EU Directives require that hospital blood banks implement a quality management system. To maintain a high level of performance in the laboratory, it is essential to monitor the functioning of reagents, equipment, techniques and procedures. Good record keeping and documentation, use of standard operating procedures and laboratory worksheets, and implementation of safety guidelines further improve the quality of performance.
The hospital blood bank is responsible for:
- Rapid response to urgent requests for blood components
- Checking pre-transfusion samples and requests
- Assessing of immunological compatibility between donor and patient
- Selecting of suitable blood component for each clinical condition
- Safe delivery and handling of blood components
- Inventory and stock management
- Interactions with the blood establishment.
Urgent Requests
All urgent requests for blood components and blood products should be notified to the laboratory by telephone. Blood bank staff should be given as much notice as possible to organise the work and assign appropriate priority to requests. The handling of any emergency situation benefits from clear and frequent communication with the hospital blood bank about the blood component requirements. A full crossmatch will take approximately 40-45 minutes from receiving the patient sample and request. In very urgent cases the time can be reduced to 20 minutes. This allows tests to exclude ABO incompatibility. In extremely urgent critical situations where blood is needed in less than 20 minutes, non-crossmatched group O blood should generally be made available for immediate use. Females of childbearing age should receive group O RhD negative red cells if the patient’s RhD type is not known.
Blood sampling and clerical checking
In pre-transfusion testing, careful checking is essential. Correctly identified and correctly labelled blood samples from the correct patient are fundamental to the provision of blood that is safe for transfusion. When a sample is received in the blood bank, a member of the staff must confirm that the information on the label and on the transfusion request are identical. The patient’s serological and transfusion history must also be checked and the results of current testing compared with those of previous tests. Any discrepancies must be resolved before any blood component can be released for transfusion.
Pre-transfusion testing
This involves testing the blood of the intended recipient to determine the ABO group and RhD type and to detect any clinically significant red cell antibodies (this procedure may be called “group and screen” or “type and screen”). If the screening test is positive further tests may be needed to identify the red cell antibodies so that compatible donor units can be selected. The patient’s serum is directly tested in the blood bank for compatibility with the donor red cells before transfusing RBC components (crossmatch). Some countries also require a further blood group check immediately before the blood is transfused.
Electronic issue (computer crossmatch)
Red cell units that are ABO and RhD compatible can be quickly issued for a patient on the basis of information in the blood bank information system, with no further testing, provided there are procedures in place to ensure that:
The patient’s ABO and RhD type have been tested and also confirmed on a second sample, retested on the first sample, or the patient has been found to be group O in the first instance
- The patient has no irregular red cell antibodies
- The grouping of the blood units is fully reliable
- The identification of the patient and his/her sample is fully reliable
- The patient’s previous results can be correctly identified and retrieved
Electronic issue can take as little as 10 minutes. Hospitals using electronic issue must comply with any applicable national guidelines.
Selection of blood component
The hospital blood bank will use the test results together with the information provided on the request form to select and label the correct blood component for the patient.
Safe delivery and handling of blood components
Errors at this stage of the clinical transfusion process are an important source of adverse reactions and events. Hospitals should have a policy that ensures that correct units are withdrawn from the storage location. Blood must only be stored in designated blood storage refrigerators with temperature monitoring charts and an alarm system.
Traceability
EU Commission Directives 2005/61/EC and 2002/98/EC (2005), require full traceability of blood and blood components, from donor to recipient and vice versa. Blood establishments and hospitals must have a system that permits identification of each unit of blood component and its final destination. A system that has proved effective in the UK is the so-called ‘bag & tag’ label system. (Fig 6.3) When a unit of blood component is prepared for a patient, a paper tag is printed from the laboratory computerised system. This includes patient identifying information and two traceability labels bearing the donation number.
The tag is attached to the unit of blood component until it is transfused (or returned to the laboratory if unused). If transfused, one label from the tag is placed in the patient’s notes and the other returned to the hospital transfusion laboratory. The data from the returned labels is entered into the computerised system that records the fate of each component. Instances of nonreturned labels are monitored and corrective action taken. Many hospitals report 95% or greater traceability using this system.
Inventory and stock management
The hospital blood bank is responsible for management of the hospital’s blood stock. This includes maintining an inventory for each blood group, ensuring an average age of blood at time of issue, and monitoring the amount of blood that becomes out dated or is not used for other reasons. Stock levels should be set in conjunction with weekly use and activity in order to avoid overstocking and wastage. Where possible an information technology (IT) system should be in place that supports blood stock management and provides a full audit trail of all blood stock electronically scanned onto the system.
The hospital blood bank should develop a partnership working agreement with their Blood Establishment provider on how to deal with shortages of blood.
Maximum Surgical Blood Order Schedule (MSBOS)
A Maximum Surgical Blood Order Schedule is a hospital policy agreed between the blood bank, clinicians and hospital transfusion committee. It specifies the number number of units of blood that should normally be crossmatched for planned surgical procedures. It takes account of the likely need for transfusion and the response time for receiving blood following a request. An MSBOS should reduce blood bank workload by avoiding unnecessary crossmatching and should contribute to stock management and reduced wastage. It is agreed through consultation between the blood bank, clinicians and hospital transfusion committee. For procedures where electronic issue is used, there is no need for the MSBOS.
9. Audit
What can audit provide?*
Audit can benefit the care of patients by stimulating review and improvement of the way things are done. It is only useful if it leads to action for improvement. Audit can improve understanding of current practice, organisation or management (descriptive audit), give information about compliance with guidelines (compliance audit), or give information about the cause of an identified problem (diagnostic audit). It can reveal good practice, providing examples of better ways of working. 15851633, A useful guide to audit is downloadable from bottom of this page.
* Words used in this chapter (see glossary)
Clinical Audit: An evaluation method that enables the comparison of practices to established references, e.g. guidelines, using precise criteria, with the aim of measuring and improving the quality of practice. (France) A method to measure the gap between ideal practice (determined from evidence and guidelines) and actual practice. (UK).
Clinical Guideline: A document developed through the consensus process describing criteria for a clinical/medical practice.
Criterion: A principle or standard by which something may be judged or decided.
| Attachment | Size |
|---|---|
| 64.3 KB |
9.1 Success factors: resources, leadership and management support
Conducting even a small clinical audit and carrying out a plan of improvement needs resources and the commitment of all participants (management, clinical teams and audit department) to complete the process. Audits that are restricted to a few patients in a single clinical unit and using minimal resources may be valuable in improving practice. Large, multi centre studies require substantial planning and resources. Such large studies may be needed to identify, on a multi centre or national scale, current practices or areas needing improvement.
9.2 Clinical audit and clinical research
Research creates new knowledge about best practice that should be used to improve guidelines. Clinical audit examines actual practice, compares it with guidelines, and tests compliance with them (example downloadable below).
| Attachment | Size |
|---|---|
| 26.96 KB |
9.3 The audit cycle
Even the best guidelines or SOP’s are only useful if they are followed. Audit is the way to test compliance. Clinical Audit should be part of a continuous improvement process or quality improvement cycle consisting of the following steps:
Choose the target
Plan to audit a topic that is clinically important, with evidence of room for improvement such as errors, adverse events or reactions, large variations in practice or patient complaints (downloadable below)
Define the aim
There should be a clear ‘audit question’ (or questions), just as any research proposal should start with a concise statement of the research question or hypothesis to be tested (downloadable below).
Select the criteria
Local criteria (developed by the hospital’s clinicians) should be used as the basis for audit. Such locally developed guidelines should be based on current national recommendations. These take account of the best available evidence.
Define the methods
Decide what is to be observed or measured, how the data will be collected, quality controlled, analysed, and presented.
Implement an action plan for improvement
Decide what will be done to improve practice if the audit shows that improvement is needed, plan and implement (downloadable below).
Repeat the audit
Test for evidence that practice has improved.
| Attachment | Size |
|---|---|
| 128.44 KB | |
| 52.8 KB | |
| 46.94 KB | |
| 36.65 KB |
9.4 Planning and setting up the clinical audit
Use an existing design
It may be possible to save time and work by using a pre-existing set of audit questions and tools, modified if necessary. This also makes it easier to compare results between institutions. Even if an existing design is used, a small pilot study should still be performed.
Design a new audit
Build the frame of reference, which should be based on the most recent, available, relevant documentation, including:
- Regulations: European directives, national laws and decrees
- Professional documentation: Clinical guidelines, consensus conferences, scientific literature, expert opinion.
- International (ISO, EN), national or professional standards
Select criteria
A critical step is to reach agreement on the criteria against which practice is to be audited. Although guidelines for many aspects of transfusion process exist, they are frequently based on inadequate evidence. There may be local barriers – such as individual clinicians’ opinions – to general acceptance of a guideline. The process of negotiating an agreed and measurable objective standard of care that can be endorsed by all stakeholders and used in the audit process can be an extremely valuable means of encouraging clinicians to review their practice (R29 document attached below).
Prospective or retrospective?
Prospective audit is based on the collection of information about patients during their process of care. It permits more reliable and complete clinical data collection since the data required is pre-defined and can be validated and errors corrected while the data collection is in progress. A possible disadvantage is that practice could be altered if staff are aware that they are being observed and data is being collected.
Retrospective audit is generally based on review of records of discharged patients. This may provide information that is more representative of day-to-day practice, but it is more difficult to obtain complete data on every subject in the sample. Retrospective audit may make use of computer databases provide the data they contain is of adequate quality.
Develop the audit criteria
A criterion is a principle or standard by which something may be judged. Audit criteria should describe the aspect of care that is being measured. Explicit means SMART:
- Specific: Unambiguous. Relates to a specific area of care and states specific boundaries
- Measurable: There must be objectively measurable aspects to allow comparison
- Achievable: Must be achievable either within available resources, availability of cases, etc.
- Research based: Wherever possible, there should be sound research evidence that shows the best available treatment or method for the aspect of care being audited
- Timely: Criteria should reflect current practice
Each criterion included in the frame of reference should ideally be derived from the reference guidelines or regulations. It should be designed to identify clearly whether practice conforms or does not conform to the guideline or regulation.
Write the protocol
The protocol should include:
- Objectives
- Sources of information used to build the frame of reference
- Definition of the audited hospitals, teams or individuals
- Criteria for inclusion and exclusion (when patients’ files are concerned)
- Type of study (prospective, retrospective)
- Type of data collection (observation by external auditor, self-evaluation, interviews, data collection from patients’ files, from hospital blood bank and from blood transfusion establishment.)
- Description of the role of each person involved.
- Any requirements related to consent, confidentiality or ethical issues
Develop or adapt tools for data collection
Data collection must be simple to perform, valid and reliable.
Data collection forms (often called Case Report Forms or CRF) must have a unique identifier, clearly identify the person who completes the document, and show the date of completion. Questions should require a simple unambiguous response (e.g. YES / NO/ Information not available). Responses such as ”not applicable” should not be permitted. The units in which quantitative results are recorded must be stated e.g. “Hb concentration to be recorded as g/l”. Clinical terms must be defined explicitly (e.g. “bleeding”, “cardiovascular disease”). Questions that require free text response should be avoided or strictly limited as they are difficult to analyse (R24 document attached below).
User manual
For a simple audit the user manual should be short and simple, explain exactly how data collectors should obtain the data items for the audit, how they should enter the information in the case report form or equivalent, and how completed CRFs should be submitted to the audit team.
Statistical support
It is strongly advised to engage the help of a statistician right from the planning stage and throughout the audit to provide expert advice on design of the study including aspects such as sample size, power, sampling methods, development of the analysis plan, conduct of the analysis and presentation of findings. If the results of the audit are to change practice, the results and analysis must be robust and also simple enough for everyone in the care process to understand.
The statistician should generally be a co-author in any report prepared for publication (R23 document attached below).
Sample size
Where a research study will need large numbers of subjects to show which intervention is best, clinical audit only needs to determine the extent to which practice complies with standards or criteria. Smaller sample sizes can often provide the information. The information collected from the audit sample should be representative, i.e. should allow 95% confidence that the results will be within 5% of the results that would be obtained from the relevant population. Sample size calculators are useful when determining an appropriate sample size (R31 document attached below).
Sampling
There are several methods of choosing which cases to include in an audit, including:
Random Sampling: Assumes your audit population will remain the same throughout the audit period and that each subject will have an equal chance of being chosen, either by drawing names out of a hat or choosing every nth subject from a list (e.g. choosing every 3rd or 5th patient).
Interval Sampling: Assumes your audit population will change over the period of the audit. In these circumstances, the audit sample is often determined by a period of time e.g. all patients transfused during May and June.
Stratified Sampling: Is a method used to ensure that the proportions of different groups in the population are reflected in the sample. For example, if investigating donor deferrals, and if male blood donors make up 40% of the donor population, you would ensure that 40% of your sample are male.
Rapid Cycle Sampling: Small data sets are audited to improve and monitor care. This approach can make the change cycle quicker and is useful if a problem is suspected and results are needed quickly. Auditing a small sample can show the nature of the problem. After implementing the action plan for improvement, a repeat the audit on another small sample can quickly show if improvement has been achieved.
Because a poorly chosen sample can skew results and give inaccurate information, the advice is repeated to seek advice from statistician or audit department (R31 document attached below).
Pilot testing
The data collection process should always be pilot tested before the full implementation of the audit. This will often lead to improvements in the data collection forms and improve the final result.
| Attachment | Size |
|---|---|
| 49.47 KB | |
| 47.39 KB | |
| 59.91 KB | |
| 104.71 KB |
9.5 Collect data
The audit team is responsible for:
- Informing all personnel involved in the audit
- Ensuring there is a clear agreement about roles, responsibilities, and authorship of the final report and any publications that result from the audit
- Training data collectors
- Providing the tools for data collection, e.g. paper case report forms with subsequent input of data to computer, PDA or other portable device
- Quality control of the collected data and of the data input process
- Ensuring anonymity of patients, audited staff or institutions when required by protocol
- Being available to respond quickly to questions and problems that arise during the audit
- Ensuring deadlines are met
(R24 downloadable below)
| Attachment | Size |
|---|---|
| 47.39 KB |
9.6 Analyse audit data
The type of analysis depends on the type of information collected. Quantitative data is concerned with numerical or specific data. E.g. Yes/No, Age, Gender, Blood Pressure, Blood Groups. The analysis of this type of data is performed using simple mathematical techniques. Qualitative data is usually descriptive rather than numerical. E.g. comments on questionnaires, or donor complaints. This data needs to be analyzed differently using specialized techniques (R23 downloadable below)
.
| Attachment | Size |
|---|---|
| 59.91 KB |
9.7 Present the results
Each team audited must have the opportunity to participate in the analysis and to study and comment on the results, express their opinions on the audit, identify causes for non-compliance and propose improvement actions. The team leader should generously acknowledge the contribution of all participants. At this stage, the team leader should be ready to provide a final validated report at the institution level. If a report is to be submitted for journal publication the active contributors should be properly recognised (R28 downloadable below).
Tables and graphs to present the results should be as simple as possible. Presentation should focus on the quality and completeness of participation and compliance with audit instructions (rate of non evaluable answers, etc.), identification of the major positive points and of the major points of nonconformity that will require improvement. It is important, at this stage, to propose a preliminary root cause analysis, in order to stimulate discussion among the participants; for each nonconforming item:
- Identify the nature of the problem
- Identify the possible causes for non-compliance
- Propose a classification to help build an action plan
- Propose an improvement action plan for consultation and agreement
(R28 downloadable below)
Search for improvement
Analysis of the audit results should define the improvements that can be proposed to the audited teams and to management. The action plan must define the objectives and the approaches to be used.
Examples
The plan may aim to improve deficiencies in the design of process or the resources that are revealed by the audit. This might involve developing or updating an SOP that is (Table 9.1) missing or out of date or correcting deficiencies in resources or training.
Table 9.1 Responding to audit findings. Examples: deficiencies in processes or resources
| Responding to audit findings – deficiencies in processes or resources | |
|---|---|
| Criterion | National and local guideline requires that hospitals have a validated procedure for provision of blood to patients in an emergency |
| Audit finding | Hospital does not have a major haemorrhage procedure |
| Corrective action | Take steps to ensure that staff who provide this service are supported by written procedures, effective training, and appropriate practises (fire drills) to test the procedures periodically |
| Criterion | Staff must receive appropriate training for their task(s) |
| Audit finding | Audits may uncover deficiencies in education and training in any area of practice |
| Corrective action | Develop and implement training programme |
| Criterion | National and local guideline requires perioperative monitoring of patients’ haemoglobin levels |
| Audit finding | Equipment for “near patient” measurement of haemoglobin concentration is not available |
| Corrective action | Operating departments must be supplied with suitable equipment |
Alternatively the audit may show that there are non-compliances even though all the appropriate procedures, personnel, training equipment, etc. are in place. (Table 9.2)
Table 9.2 Responding to audit findings. Examples: non-compliance
| Responding to audit findings – non-compliance | |
|---|---|
| Criterion | Guidelines require that patient records contain a record of the clinician’s reason for prescribing each red cell transfusion. |
| Audit finding | A doctor’s record of the reason for transfusing is found in only 20% of patients’ files. |
| Corrective action | Obtain the agreement of clinical staff to achieve a target of 90% documentation of the reason to transfuse and to participate in education on the importance of clinical accountability for transfusion and to a repeat audit. |
| Criterion | Guidelines require that all patients undergoing transfusion have observations of pulse, blood pressure, respiration and temperature recorded before and at specified time intervals during the transfusion. |
| Audit finding | These “routine observations” are performed incompletely or not at all in a substantial proportion of transfusion episodes. |
| Corrective action | Obtain the agreement of clinical staff to achieve a target of 90% documentation of patient observations according to the guidelines and to a repeat audit. To overcome the problem that nursing staff believe that they do not have time to perform the task, consider action such as:
|
| Criterion | EU Directive requires that the final fate of all blood components issued for recipients is recorded by the hospital blood bank. |
| Audit finding | Hospital blood bank does not have data on the final fate of all components |
| Corrective action | Obtain the agreement of clinical staff to achieve an initial target of 98%. Inform staff that monthly reports will be provided to senior nursing managers, identifying the clinical areas not meeting the agreed target. These mangers will be required to identify how non-conformance will be addressed. |
| Attachment | Size |
|---|---|
| 43.57 KB |
9.8 Presentation of results
Practical proposals for corrective measures must be made to the hospital management. These must be endorsed by all participants in the audit. The presentation should identify both positive and negative findings of the audit, since sharing examples of good practice may contribute as much to improving quality as does the identification of inadequate practice. The improvements expected from carrying out the action plan should be described as accurately as possible. The improvement action plan should be finalised and approved by the project team, the audited team and the hospital management during or shortly after this presentation.
The Audit report
The final report should present the overall project and should include the following sections:
- Objectives
- Participants (Project team, Audited teams)
- Organisation and methodology
- Frame of reference and timeline from planning to report
- Both positive findings and issues requiring improvement
- Agreed action plan for improvement
- Annexes (documents used, e.g. audit protocol, user manual, reference sources)
Publication in the professional literature may add greatly to the value of the audit, for the participants, the hospital and the wider professional community.
9.9 Detailed examples of audits
Practical examples of audit tools and reports can be found in the resources listing and in the file list below.
| Attachment | Size |
|---|---|
| 56.2 KB | |
| 183.56 KB | |
| 50.83 KB | |
| 107.84 KB | |
| 3.41 MB | |
| 112.46 KB | |
| 71.52 KB | |
| 210.55 KB |
10. Training
Directive 2005/62/EC (Annex: 2.1) requires that personnel in Blood Establishments shall be trained and assessed to be competent in the their tasks. This chapter provides an introduction to some of the practical issues that are likely to be encountered if this requirement were to be applied – for example to comply with a national regulation - to all staff who have a role in the clinical transfusion process.
Some of the challenges that may have to be overcome to provide effective training and assessment for hospital staff are:
- The large number of individuals and the range of different employment groups who have some involvement in the process
- Rapid turnover of staff ( for example due to training requirements for clinical staff)
- Working patterns – shiftwork, part time work
- Multiple employers – eg agencies providing nurses and doctors, external contractors for support services
- Language differences due to mobility of personnel within the EU
- Differences in education, training and the details of practice experience of personnel from different member states
Education and training is fundamental to every aspect of blood transfusion safety. The development of guidelines and S.O.P.’s is insufficient to alter clinical practice: they must be used. As well as meeting EU requirements, it is important to comply with any national requirements for training staff involved in transfusion. Although every EU country will have different access to resources and facilities, there are a number of essential steps to consider when implementing an education and training programme in transfusion.
Figure 10.1 Example of the steps for implementing an education and training programme
10.1 Establish leadership and management support
An effective training programme requires leadership and commitment from the senior management of the organisation. They need to be aware of the regulatory requirements of the EU Blood directives together with other national standards for safe and appropriate transfusion practice.
It is essential to have an active multidisciplinary Hospital Transfusion Committee (HTC) that takes responsibility for developing and implementing a strategy for the education and training of all clinical, laboratory and support staff involved in blood transfusion. A lead person should be appointed to oversee the day-to-day running of the training programme and must have access to adequate staffing and resources.
10.2 Undertake training needs assessment
Who needs training in what?
Various staff groups have been identified as involved in the process for transfusing blood in hospitals. Undertaking a Training Needs Assessment (TNA) will help to determine:
- The knowledge and skills requirements for each specific task in the clinical transfusion process
- Which staff groups require training
- How many in each specific staff group require training
- What training is currently available and who is responsible for training of each of the staff groups
- A baseline as a basis for later progress reporting
A template for training needs assessment is provided on the website.
Trainers
It is also essential to review the training needs of the trainers to support and develop their ability, confidence and motivation to deliver effective teaching. Trainers need to maintain their knowledge by continuous professional development. They should have access to courses and opportunities for self-learning. In addition, they should have access to training courses to develop and maintain other specific skills in communication, IT, etc.
10.3 Baseline assessment of knowledge and practice
It is helpful to have data on transfusion practices before the implementation of the education programme. Transfusion practice audits, and reviews of errors and near misses reported to the HTC or the haemovigilance system will provide valuable information on where training and education should be targeted.
The practices that should be audited derive from the activities identified in the essential steps in the clinical transfusion process. (Fig 2.1)
Member States may have different names for similar jobs, and some job titles will not exist in some countries. There are differences among member states and local hospitals as to which staff group undertake particular tasks.
Figure 10.2 identifies the knowledge required for each task. Areas of knowledge and procedures that should be evaluated are:
- Knowledge of blood group serology
- Knowledge of characteristics of blood components
- Procedures for blood sampling and labelling
- Procedures for storage of blood components
- Procedures for collection and delivery of blood components
- Checking and administration procedures for blood components
- Procedures for monitoring the transfused patient
- Understanding of adverse events in transfusion
It is essential to make a realistic assessment of the resources (personnel and financial) required for collecting baseline data and for an ongoing audit programme to ensure standards are being maintained. Each hospital should have a clinical audit department (or a similar function as part of quality management) that should be able to provide advice.
Two methods often used to gather information about existing knowledge and practice are questionnaires and observation of practice.
Questionnaires should reflect the required standards of practice and may differ for each group of staff. While a questionnaire can be a relatively simple way to obtain information, there are known problems. These include poor response rate, deficient completion and the temptation to give the ‘correct’ rather than a ‘true’ response.
Observational audit of transfusion practices can yield very useful information, but is labour intensive and difficult to undertake. Direct observation can make staff alter their practice, however there is evidence to suggest that staff become used to the observer and continue with their usual practice.
Figure 10.2 Essential knowledge and skills for the clinical transfusion process
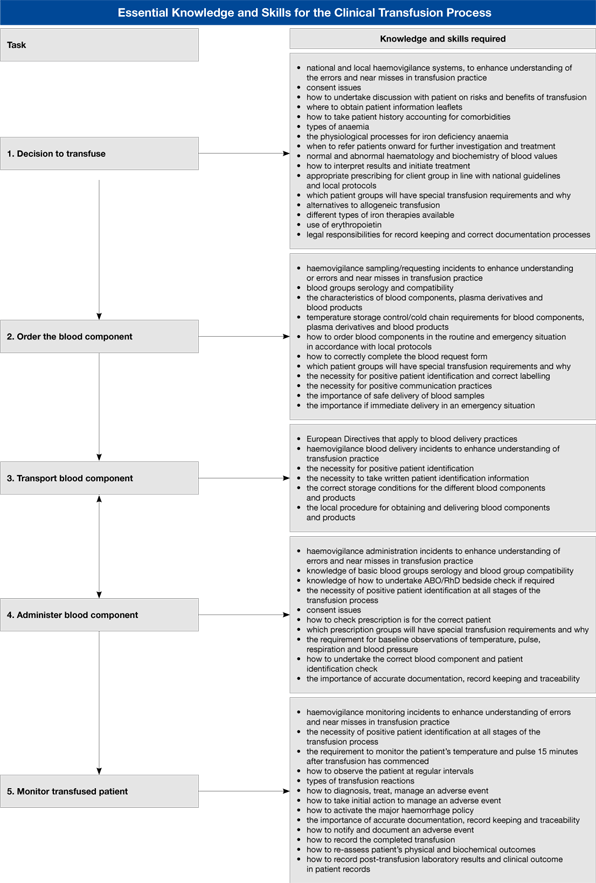
10.4 Obtain teaching and training material
If you choose to develop your own materials, this will require careful planning and dedicated time. All learning materials should be critically reviewed by subject experts. Due to the large numbers of staff involved in blood transfusion practice, e-Learning education programmes in transfusion have been developed and may be useful in the training programme. However, e-Learning should not be seen as an easy answer, since it requires a comprehensive support strategy.
This must cover the following:
- Access to computers for staff
- Connectivity and bandwidth
- Security of personal information
- IT skills of the learner (consider providing the first e-Learning experience in a facilitated environment)
- Provision of a help line for users and technical support for learners
- Providing step-by-step user guides
- Dedicated time for training.
- Setting up e-learning champions
To access the majority of these e-Learning education programmes, the learner will need an email address and Adobe Flash Player version 8 (or higher). Some English languagelearning sites are given below. See also the list of links at the end of the manual.
Better Blood Transfusion - Continuing Education Programme
www.learnbloodtransfusion.org.uk
Bloody Easy Online Course
http://sunnybrook.nextmovelearning.com
Blood Safe Online Transfusion Course
http://www.bloodsafelearning.org.au/
Learn Cell Salvage
http://www.learncellsalvage.org.uk/
Nursing CE: Blood and Blood Product Administration
www.elearners.com/course/31266.htm
10.5 Select teaching methods
There are several different teaching methods that may be of use in delivering transfusion education. The choice will depend on the target group, the numbers that require transfusion training and the level of training required. Table 10.1 gives a brief description of some of methods.
Table 10.1 Teaching methods
| Method | Description | Pros and cons |
|---|---|---|
| Large group teaching — lectures | Historically the most widely used teaching technique. Very useful for providing training to large numbers of learners who need the same information. Can be supported by handouts to promote call of information. | Inexpensive approach, however, the quality of lecture is dependent on the knowledge, skill and attitudes of teacher, and learners may feel they have a ‘passive’ role with lack of involvement. |
| Small group learning | An interactive learning approach using small group, problem based learning. The trainer has the role of facilitating, prompting and providing guidance and prompt feedback. Medical undergraduate education has moved to this approach in may countries. | This method can be used for multidisciplinary education for key staff involved in transfusion. Promotes active participation, sharing of experiences, and learning from each other. |
| Individual learning | Learning can be self-directed using paper based materials or e-Learning. Should not be used in isolation but integrated into the wider programme. Requires a clear strategy with standardisation of approach. | Learners must have key IT skills and access to IT resources if using e-learning packages. Individual learning is unsuitable for developing practical transfusion skills. |
| Simulated learning | This technique has been adapted for use in healthcare. Can be used to recreate common errors in transfusion practice e.g. ‘wrong blood’ incidents. | Expensive and only suitable for training small numbers per session. |
10.6 Assess theoretical knowledge and practical competency
Directive 2005/62/EC requires that in blood establishments, competence of personnel shall be evaluated regularly (Annex: 2.4). If this principle is to be extended to cover all staff involved in the clinical transfusion process, it will be necessary to consider the points that follow.
The purpose of assessment is to evaluate or measure achievement of learning and competence, and provide information for more effective teaching. There are four stages of development that an individual progresses through from acquiring knowledge to performing a task in clinical practice and these are “knows, knows how, shows how and does”, and each level requires to be assessed differently. See Figure 10.3
Level 1 and 2 theoretical competency
A number of methods can be used to assess the retention of theoretical knowledge following training. These can be paper based or part of the e-Learning programme. The advantage of the e-learning approach is that assessments are scored and recorded online, avoiding time-consuming traditional methods.
Level 3 and 4 practical competence
Formal assessment of clinical competence can be used to integrate theory with practice. Level 3 and 4 are difficult to assess. Issues that have been identified in the UK during the introduction of competency assessment for the clinical transfusion process are:
- The large number of individuals to be assessed.
- Dedicated preparation time is required for the assessor.
- Time must be allocated for the staff to be assessed
- Difficulty in finding clinical situations for assessment
- Cost
Tools for assessing practical competency are available from several organisations: examples of English language versions can be found at the sites below:
http://www.npsa.nhs.uk/patientsafety/alerts-and-directives/notices/blood-transfusions
http://www.skillsforhealth.org.uk
A description of the methods that can be used assess theoretical and practical competency is provided in table 10.2.
Table 10.2 Assessment of knowledge and competency
| Method | Description |
|---|---|
| Background Knowledge Tests | Short, simple questionnaires for use prior to implementing a training programme or introducing an important new topic. |
| Multiple-choice questions (MCQ) | Measures both simple knowledge and complex concepts. MCQ can be answered quickly and can be easily and reliably scored. |
| True-false questions | Are less reliable because random guessing may produce the correct answer. However, they provide a method for recall and can be easily and reliably scored. |
| Matching tests | An effective way to test learners’ recognition of the relationships between words and definitions and categories and examples. |
| Checklist evaluation | Useful for evaluating any competency that can be broken down into specific behaviours, activities or steps that make up a task or procedure. Can also be used for self-assessment of practice skills. |
| Objective structured clinical examination (OSCE) | Assessments are administered at a number of separate standardised patient encounter stations. Each station lasting 10-15 minutes. |
| Live simulated situation | Imitate but do not duplicate real life situations. ‘Actor’ patients or mannequins can be used and scenarios can be administered individually or in groups. They are resource intensive however, and the assistance of technical expertise is required. |
| Computer simulation | Expensive to create, however, provides an opportunity to assess skills without possible harm to live patients. There is exposure to standardised training content and the ability to provide immediate feedback to the learner. |
| Direct observation of practice | Assessment takes place in a real practice setting. Desired or proficiency required in specific behaviours in conducting skill have to be demonstrated. |
| Videotaping a practice session | Seen as a poor assessment technique however, as it captures performance and not competence. |
10.7 Manage training records
Records of training and assessment of B.E staff are required by Directive 2005/62/EC. Suitable records would show for each person that the required training, assessments and updating had been undertaken. A training record should, as a minimum, contain the following information:
- Name of trainee
- Unique identification number
- Place of work
- Date of training session
- Type of training session
- Length of time of training
- Training method
- Name of the trainer(s)
- Evaluation method
- Attainment achieved
- Record of assessment of competence
These principles would also apply to training records for staff involved in the clinical transfusion process.
10.8 Evaluate the training programme
The evaluation of the teaching programme against predetermined goals can help determine the overall effectiveness of several components. This includes participant learning, trainer effectiveness, learning environment, use of resources and organisational impact. The main areas of importance are:
- Educational outcomes — has understanding and retention of knowledge following a training session improved?
- Clinical outcomes — has occurrence of transfusion critical incidents or specific aspects of transfusion practice improved (e.g. patient observation during transfusion, documentation of indication for transfusion in case notes etc.)?
- Qualitative and quantitative feedback by trainees and trainers — can be used to evaluate particular teaching sessions. Questionnaires can be paper based or electronic.
Evaluation by trainees
Areas to evaluate:
- Facilities used for training e.g. venue, access to venue for face-to-face teaching
- Access to IT resources for computer based learning
- Did the teaching session cover pre-defined key learning objectives
- Likely impact or anticipated change in clinical practice
- Quality and content of teaching material
- Quality and content of any handout material
- Quality of teaching method
- Clarity of presentation
Evaluation by trainers
Areas to evaluate:
- Facilities available for training e.g. venue etc
- Key learning objectives clearly defined
- Clear information available regarding training needs of target group
- Training provision for teaching and facilitation skills (‘training the trainer’)
- Resources available for teaching
- Quality of teaching material (learner feedback on slides, handouts etc)
- Accessibility to training (e.g. computer based learning)
10.9 Sustain the momentum of the programme
It is recognised that immediately following training, staff demonstrate higher levels of awareness, motivation and performance, but in time this may decline and bad habits may return to reduce the quality of the work. Suggestions for achieving commitment and maintaining momentum between training sessions are:
- Maintain regular communication with staff
- Be visible
- Use hospital newsletters, hospital intranet, informal teaching/lecturing sessions to promote e-Learning programmes and other learning opportunities
- Set up a network of interested individuals in clinical areas to help disseminate information
- Make sure that protocols and guidelines are available to all the people that should be using them
- Use early feedback to clinical areas of transfusion events or reactions, and lessons learned
- Encourage trainees/learners to provide feedback on training
Implementing a transfusion training and education programme can be very challenging. Finance and facilities may be inadequate to meet the training needs of a large diverse group of staff. Strong and sustained support from management, backed with resources for necessary people and materials is essential.
Table 10.3 Teaching methods
Triangle of knowledge/ clinical skills competence/performance (Adapted from Miller 1980)
11. Resources
Glossary
| Term | Definition | Source / Defined in |
|---|---|---|
| Additive solution | A solution specifically formulated to maintain beneficial properties of cellular components during storage | Directive 2004/33/EC |
| Administer | Used in the manual to mean “administer a blood transfusion”, “give a blood transfusion” | EU OBU Project team |
| Adverse event (serious adverse event) |
Any untoward occurrence associated with the collection, testing, processing, storage and distribution of blood and blood components that might lead to death or life threatening, disabling or incapacitating conditions for patients or which results in, or prolongs, hospitalisation or morbidity | Directive 2002/98/EC |
| Adverse reaction |
|
Steadman’s Medical Dictionary 2002 |
|
Directive 2002/98/EC | |
| Allergic reaction | One or more of: rash, dyspnoea, angioedema, generalized pruritis, urticaria, without hypotension within 24 hours of transfusion | IHN http://www.ehn-net/Portal.aspx |
| Allogeneic donation | Blood and blood components collected from an individual and intended for transfusion to another individual, for use in medical devices or as starting material/raw material for manufacturing into medicinal products | Directive 2004/33/EC |
| Alloimmunization (due to transfusion) | Formation of antibodies to RBC, HLA, HPA and HNA antigens which were not detectable pre-transfusion | IHN http://www.ihn-org.net/Portal.aspx |
| Anaphylactic reaction | Hypotension with one or more of: rash, dyspnoea, stridor, wheezing, angioedema, pruritus, urticaria, during or within 24 hrs of transfusion. | SHOT http://www.shotuk.org/ |
| Anticoagulant solution | A substance that prevents or delays blood clotting (coagulation) | http://www.transfusionguidelines.org.uk |
| Audit |
|
www.eubis-europe.eu |
|
EU OBU Project team | |
| Audit (prospective) | An audit in which the participants are identified and then followed forward in time | http://www.merriam-webster.com/dictionary |
| Autologous donation | Blood and blood components collected from an individual and intended solely for subsequent autologous transfusion or other human application to that same individual | Directive 2004/33/EC |
| Benchmark | Something set up as an example against which others of the same type are compared | www.merriam-webster.com |
| Blood | Used in this manual as a short form of “blood and blood components | EU OBU Project team |
| Blood bank (hospital blood bank) | A hospital unit which stores and distributes and may perform compatibility tests on blood and blood components exclusively for use within hospital facilities, including hospital based transfusion activities | Directive 2002/98/EC |
| Blood establishment | Any structure or body that is responsible for any aspect of the collection and testing of human blood or blood components, whatever their intended purpose, and their processing, storage, and distribution when intended for transfusion. This does not include hospital blood banks | Directive 2002/98/EC |
| Blood component | A therapeutic constituent of blood (red cells, white cells, platelets, plasma) that can be prepared by various methods intended for transfusion | Directive 2002/98/EC |
| Blood donation | See “donation” | |
| Blood group | An immunologically distinct, genetically determined class of human blood that is based on the presence or absence of certain red cell antigens and is clinically identified by characteristic agglutination reactions or by nucleic acid testing | EU OBU Project team |
| Blood product | Any therapeutic product derived from human whole blood or plasma | Directive 2002/98/EC |
| Blood transfusion chain | The numerous activities that occur from the moment an individual offers to donate blood or plasma until after the blood product has been administered to the patient | www.eubis-europe.eu/ |
| Clinical audit |
|
National Institute of Health and Clinical Excellence in the UK www.nice.org.uk |
|
French Health Authority ‘Haute Autorité de Santé’ www.has-sante.fr | |
|
EU OBU Project team | |
| Clinical Guideline |
|
Scottish Intercollegiate Guidelines network (SIGN) |
|
Canadian Blood Service http://www.bloodservices.ca/s |
|
|
http://www.transfusion.ca/ | |
| Collect, Collection | Usage in this manual: Process in which blood components are collected from the hospital blood bank or from a satellite refrigerator prior to transfusion. This usage should be distinguished from “collection of a donation from the donor” | EU OBU Project team |
| Compatibility report | Report that accompanies the blood components that are issued by the hospital blood bank for transfusion to a recipient | EU OBU Project team |
| Compatibility test | Laboratory test performed to ensure immunological compatibility between donor blood and prospective recipient’s blood or blood components prior to transfusion | EU OBU Project team |
| Competency | Ability of a person to perform a specific task according to procedures | www.eubis-europe.eu/ |
| Consent | To give assent or approval (e.g. consent to being transfused) | http://www.merriam-webster.com/dictionary |
| Criterion | A principle or standard by which something may be judged or decided | Ask Oxford.com Compact Oxford English dictionary |
| Deliver, Delivery |
|
American Heritage Dictionary of the English Language |
|
EU OBU Project team | |
| Distribution | The act of delivery of blood and blood components to other blood establishments, hospital blood banks and manufacturers of blood and plasma derived products. It does not include the issuing of blood or blood components for transfusion | Directive 2002/98/EC |
| Document |
|
ISO 9001 www.iso.org |
|
http://www.merriam-webster.com/dictionary/ | |
|
http://www.merriam-webster.com/dictionary/ | |
|
EU OBU Project team | |
| Donation | Something that is given to a charity. Used in the manual in the sense “blood donation” | Ask Oxford.com Compact Oxford English Dictionary |
| Disseminate | Distribute, diffuse, communicate widely | Collins English Dictionary 4th ed 1999 Harper Collins |
| E-learning | The delivery of a learning, training or education program by electronic means | EU OBU Project team |
| Educate | Train by formal instruction and supervised practice especially in a skill, trade, or profession. (Similar to: Teach: to impart the knowledge of) | http://www.merriam-webster.com/dictionary/ |
| Efficient | Working productively with minimum wasted effort or expense | http://www.askoxford.com/concise_oed |
| Elective | Beneficial to the patient but not essential for survival, as in: “elective surgery” | http://www.merriam-webster.com/dictionary/ |
| Emergency |
|
Collins English Dictionary 4th ed 1999 Harper Collins |
|
||
|
||
| EPO | Abbreviation for: Erythropoietin, epoietin | |
| ERM | Abbreviation for: Electronic Record Management | |
| Error |
|
www.eubis-europe.eu/ |
|
http://www.merriam-webster.com/dictionary/ | |
| European Commission (EC) | The executive organ of the European Union, based in Brussels, which monitors the proper application of the Union treaties and the decisions of the Union institutions | http://www.coe.int/aboutCoe |
| European Union (EU) | The EU currently has 27 members that have delegated some of their sovereignty so that decisions on specific matters of joint interest can be made democratically at European level. No country has ever joined the EU without first belonging to the Council of Europe | http://www.coe.int/aboutCoe |
| Evidence-based medicine | The conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients. The practice of evidence-based medicine means integrating individual clinical expertise with the best available external clinical evidence from systematic research | Health Services Executive, Republic of Ireland http://www.hse.ie |
| Expire | Come to the end of the period of validility | Ask Oxford.com Compact Oxford English Dictionary |
| Expiry date (blood components / products) | The last day on which the blood or blood component is considered fit for use for therapeutic transfusion purposes | ESOP Manual Ed 1.0, 2007 www.eubis-europe.eu/ |
| External review | An evaluation of the quality and effectiveness of a system carried out by a team of external evaluators who are specialists in the fields reviewed | EU OBU Project team |
| Facilities | Hospitals, clinics, manufacturers, and biomedical research institutions to which blood or blood components may be delivered | 2005/61/EC,EU-Q-Blood-SOP, |
| FFP (Fresh Frozen Plasma) | The supernatant plasma separated from a whole blood donation or plasma collected by apheresis, frozen and stored | Directive 2004/33/EC |
| Graft-versus-host disease (transfusion associated) | A generally fatal immunological complication of transfusion involving the engraftment and clonal expansion of viable donor lymphocytes, contained in blood components in a susceptible host. | Serious hazards of transfusion (SHOT), UK www.shotuk.org |
| Haematocrit | Ratio of the volume occupied by red cells to the total volume of blood expressed as a percentage | Collins English Dictionary 4th ed 1999 Harper Collins |
| Haemovigilance | A set of organised surveillance procedures relating to serious adverse or unexpected events or reactions in donors or recipients, and the epidemiological follow-up of donors | Directive 2002/98/EC |
| Haemolytic transfusion reaction (Acute) (HTR) | Acute: fever and other symptoms/signs of haemolysis within 24 hours of transfusion; confirmed by a fall in Hb, rise in LDH, positive DAT and positive crossmatch. Delayed: fever and other symptoms/ signs of haemolysis more than 24 hours after transfusion; confirmed by one or more of: a fall in Hb or failure of increment, rise in bilirubin, positive DAT and positive crossmatch not detectable pre-transfusion. Simple serological reactions (development of antibody without pos DAT or evidence of | IHN http://www.ehn-org.net/Portal.aspx |
| Hospital Blood Bank | A hospital unit which stores and distributes and may perform compatibility tests on blood and blood components exclusively for use within hospital facilities, including hospital based transfusion activities | Directive 2002/98/EC |
| Identification (of a patient) | The documented confirmation of a specified set of patient identifying information as belonging to the respective individual | Modified from ESOP Manual Ed 1.0, 2007 www.eubis-europe.eu/ |
| Imputability | The likelihood that a serious adverse reaction in a recipient can be attributed to the blood or blood component transfused or that a serious adverse reaction in a donor can be attributed to the donation process | Directive 2005/61/EC |
| Inspection | Formal and objective control according to adopted standards to assess compliance with relevant legislation and to identify problems | Directive 2002/98/EC |
| Irradiated blood components | Cellular blood component treated with 25 gray (Gy) gamma irradiation to inactivate lymphocytes that could cause graft-versus host disease in a recipient | www.transfusionguidelines.org.uk |
| Issue (blood component) |
The provision of blood or blood components by a blood establishment or a hospital blood bank for transfusion to a recipient Since the word “provision” embraces several different tasks, the Manual uses the term “delivery” to denote the physical movement of the blood component unit from blood bank to the patient’s clinical unit or operating room. |
Directive 2005/61/EC, |
| Labelling | Information that is required/ selected to accompany a product, and may include content, identification, description of processes, storage requirements, expiration date, cautionary statements, or indications for use | Quoted in: AABB (ESOP Manual Ed 1.0, 2007) |
| Leukoreduction / leukodepletion | A process used to remove white blood cells from blood components before transfusion | www.transfusionguidelines.org.uk |
| Methods | A way of doing something, especially a systematic way; implies an orderly logical arrangement | Miriam Webster online thesaurus |
| Monitor | Continuous observation and measurement of a variable, to check on a given condition | EU OBU Project team |
| Near miss | An error that might have harmed a patient but did not | BMJ 2009 |
| Order (blood) | Request that something be made, supplied or served (used in this manual in the sense) “order blood for a patient” | Ask Oxford.com Compact Oxford English Dictionary |
| Pack | In the Manual, the term “pack” is used to denote the blood component container and its contents | EU OBU Project team |
| Parameter |
|
Miriam Webster online thesaurus |
|
Ask Oxford.com Oxford Thesaurus |
|
| Patient at risk of transfusion | Patient who must be transfused OR who, with good clinical management, may avoid the need for transfusion | EU OBU Project team |
| Personnel | A body of persons usually employed (as in a factory, office, or organization) | http://www.merriam-webster.com/dictionary |
| Pilot tests | Preliminary test or study of the program or evaluation activities to try out procedures and make any needed changes or adjustments | http://www.merriam-webster.com/dictionary |
| Positive patient identification | Process in which patient is asked to give her/his details in order to ensure correct identification and subsequent administration of the right blood component | EU OBU Project team |
| Prescription Form | A form on which the clinician prescribes a medicine, or a blood component to be transfused to the patient | EU OBU Project team |
| Pre-transfusion Sampling | Procedure for taking blood samples from the patient requiring a transfusion, for compatibility investigation | EU OBU Project team |
| Pre-transfusion blood sample | The patient blood sample obtained prior to transfusion in order to assess blood group and compatibility | EU OBU Project team |
| Procedure | A procedure controls a distinct process or activity, including the associated inputs and outputs. A series of tasks usually performed by one person according to instructions | ISO9001 |
| Process | A set of related tasks and activities that accomplish a work goal AABB (ESOP Manual Ed 1.0, 2007) | www.eubis-europe.eu |
| Processing | Any step in the preparation of blood component that is carried out between the collection of blood and the issuing of blood component | Directive 2005/62/EC |
| Protocol | A detailed plan of a medical treatment or procedure Modified from http://www.merriam-webster.com/dictionary | http://www.merriam-webster.com/dictionary |
| Quality | Manufacture of medicinal products so as to ensure that they are fit for their intended use, comply with the requirements of the Marketing Authorisation and do not place patients at risk due to inadequate safety, quality or efficacy. (according to EN ISO 9000:2005; quality is defined as the ‘degree to which a set of inherent qualities are met’) | EC GMP 2006 Chapter 1 (ESOP Manual Ed 1.0, 2007)www.eubis-europe.eu |
| Recipient (of blood) | Someone who has been transfused with blood or blood components | Directive 2005/61/EC |
| Record |
|
http://www.bloodservices.ca/ |
|
www.iso.org | |
| Release | A process which enables a blood component to be released from a quarantine status by the use of systems and procedures to ensure that the finished product meets its release specification | Directive 2002/98/EC |
| Reporting establishment | The blood establishment, the hospital blood bank or facilities where the transfusion takes place that reports serious adverse reactions and /or serious adverse events to the competent authority | Directive 2005/61/EC |
| Requirement | A need, expectation or obligation. Can be stated or implied by an organisation, its customers or other interested parties. There are many types of requirements. Some of these include quality requirements, customer requirements, management requirements and product requirements | www.iso.org |
| Resources | Include people, money, information, knowledge, skills, energy, facilities, machines, tools, equipment, technologies and techniques | www.iso.org |
| Satellite refrigerators | Controlled blood storage refrigerators located remote from the main hospital blood bank | EU OBU Project team |
| Serious adverse event | See: Adverse Event | Directive 2002/98/EC |
| Specification | A description of the criteria that must be fulfilled in order to achieve the required quality standard | Directive 2005/62/EC |
| Staff | see ‘personnel’ | |
| Standard |
|
Directive 2005/62/EC |
|
http://www.bloodservices.ca/ | |
|
www.iso.org | |
| Sterile | Free from viable micro-organisms | ESOP Manual Ed 1.0, 2007 |
| Systematic review | A review of the literature on a topic that is based on comprehensive searching of all relevant sources and that employs explicit criteria to assess the eligibility and methodological quality of the studies | EU OBU Project Team |
| Tools | Something that helps you do a particular activity. Used in this manual to mean methods, techniques | Cambridge.com Cambridge dictionary online |
| TNA | Abbreviation for: Training Needs Assessment | |
| Traceability | The ability to trace each individual unit of blood or blood component derived thereof from the donor to its final destination, whether this is a recipient, a manufacturer of medicinal products or disposal and vice versa | Directive 2005/61/EC |
| Training | As work in blood transfusion chain is of a specialised nature, specific training of all staff is necessary if they are to perform their duties satisfactorily. Failure to do so will compromise the quality of products | www.eubis-europe.eu |
| Transfusion-associated autoimmune haemolytic anaemia (TA-AIHA) |
Haemolysis-related symptoms (pallor, tachycardia, hyperventilation, etc) in a temporal association with transfusion. TA-AIHA is confirmed by a drop in haemoglobin level, a positive direct antiglobulin test and an eluate revealing an erythrocyte autoantibody which was not present in the recipient’s blood pretransfusion |
http://www.ehn-org.net/Portal.aspx |
| Transfusion-associated circulatory overload (TACO) | Respiratory distress, tachycardia, increased blood pressure, typical signs of cardiogenic lung oedema in the chest x-ray, evidence of a positive fluid balance and / or a known compromised cardiac status during or within 12 hours after transfusion | http://www.ehn-org.net/Portal.aspx |
| Transfusion-associated dyspnoea (TAD) | Respiratory distress in temporal association with blood transfusion with no evidence of TRALI, allergic dyspnoea or TACO | http://www.ehn-org.net/Portal.aspx |
| Transfusion (Blood) chain | The numerous activities that occur from the moment an individual offers to donate blood or plasma until after the blood product has been administered to the patient | www.eubis-europe.eu |
| Transfusion Committee (Hospital transfusion committee) | SEE hospital transfusion committee | |
| Transfusion reaction | Any clinical reaction considered to be related to a blood transfusion event | http://www.bloodservices.ca/ |
| Transfusion related acute lung injury (TRALI) | (TRALI) Acute lung injury following within hours of a transfusion (2) A SHOT incident category and defined as: Acute dyspnoea with hypoxia and bilateral pulmonary infiltrates during or within 6 hrs of transfusion, not due to circulatory overload or other likely cause | www.transfusionguidelies.org.uk |
| Transfusion transmitted infection | The recipient had evidence of infection post-transfusion and there was no evidence of infection prior to transfusion and no evidence of an alternative source of infection; and, either at least one component received by the infected recipient was donated by a donor who had evidence of the same transmissible infection or at least one component received by the infected recipient was shown to contain the agent of infection | http://www.ehn-org.net/Portal.aspx |
| Transfusion Safety Officer | TSOs are responsible for the quality and safety of transfusion within their respective institutions, particularly in the transfusion service and in the transfusing units, wards or clinics | http://www.transfusion.ca |
| Unit (of blood component) | In the Manual, the term ‘red cell unit’ is used to denote the red cells from one standard blood donation | EU OBU Project team |
| Untoward | Unexpected and inappropriate or adverse | Compact Oxford Dictionary |
| Validation | The establishment of documented and objective evidence that the pre-defined requirements for a specific procedure or process can be consistently fulfilled. The establishment of documented and objective evidence that the pre-defined requirements for a specific procedure or process can be consistently fulfilled | 2005/62/EC |
| Warming (blood component) | The controlled increase of the temperature of blood components prior to transfusion | EU OBU Project team |
| Whole blood |
|
2004/33/EC |
|
www.transfusionguidelines.org.uk modified EU Op Lip project team |
|
| Wrong blood | Events where a patient received a blood component intended for a different patient or of an incorrect group | www.shot.org.uk |
11.1 List of contributors
Dr Guenther J Wittauer, Head of Blood Services,
Austrian Red Cross Blood Services; Head of Administration,
Austrian Red Cross Blood Donation Centre for Vienna,
Lower Austria and Burgenland, AUSTRIA
Dr Christof Jungbauer, Head of Laboratories,
Austrian Red Cross Blood Donation Centre for Vienna,
Lower Austria and Burgenland, AUSTRIA
Dr Petr Turek, Head of National Blood Transfusion Committee,
Thomayer Teaching Hospital, Prague, CEZCH REPUBLIC
Dr Jiri Masopust, Vice President of the Czech Society for
Transfusion Medicine / Head of Department of Transfusiology,
Masaryk Hospital Usti nad Labem, CEZCH REPUBLIC
Dr Lenka Walterova, Chief, Department of Haematology,
Liberec Regional Hospital, Liberec, CZECH REPUBLIC
Dr Riin Kullaste, Director of Blood Centre, North Estonia Medical Centre, ESTONIA
Dr Georges Andreu, Medical and Scientific Director,
National Institute of Blood Transfusion, FRANCE
Dr Genevieve Gondrexon, Establissement Francais du Sang Lorraine Champagne, FRANCE
Professor Christian Seidl, Vice-Medical Director,
Institute of Transfusion Medicine and Immunohaematology,
German Red Cross, GERMANY
Dr Olga Marantidou, Scientific Director,
Greek National Blood Centre, GREECE
Dr Eleftheria Zervou, Blood Bank Director,
University Hospital of Loannina, GREECE
Dr Eleni Theodori, Blood Bank Director, University Hospital of Patras, GREECE
Dr Vincenzo de Angelis, Director,
Transfusion Medicine Department, Azienda Ospedaliero-
Universitaria, ‘S. Maria della Misericordia’- UDINE (Italy)
Dr Stefan Laspina, Consultant in Transfusion Medicine,
Mater Dei Hospital, MALTA
Dr Magdalena Letowska, Deputy Director for Transfusion
Medicine, Institute of Haematology and Transfusion Medicine,
POLAND
Dr Margarida Amil, Chief of Service of Imunohemoterapia,
Centro Hospitalar do Porto, PORTUGAL
Dr Fatima Nascimento, Chief of Service in Transfusion
Medicine and member of the Board of Portuguese Blood
Institute, Portuguese Blood Institute, PORTUGAL
Dr Laura Castro, Director of the Regional Blood Centre of Lisbon, PORTUGAL
Dr Erika Deak, Assistant Professor,
Department of Physiology and Immunology,
University of Medicine and Pharmacy,
Victor Babes Timisoara, ROMANIA
Dr Alina Debrota, Director, Regional Blood Transfusion Centre
of Constanta, ROMANIA
Dr Andy Rosin, Director of Transfuziology Center, International
Collaborations, ROMANIA
Dr Dragoslav Domanovic, Director of Blood Supply
Department, Blood Transfusion Centre of Slovenia, SLOVENIA
Professor Rene R P de Vries, Head of Blood Transfusion
Service and President of the International Haemovigilance
Network (IHN), Leiden University Medical Centre,
THE NETHERLANDS
Dr Kieran Morris, Acting Medical Director, Northern Ireland
Blood Transfusion Service, NORTHERN IRELAND
Dr Simon Stanworth, Consultant Haematologist, National Blood
Authority and Oxford Radcliff Hospitals NHS Trust, ENGLAND
Dr Shubha Allard, Consultant Haematologist, National Blood
Authority and Barts and the London NHS Trust, ENGLAND
Dr Brian McClelland, Strategy Director, Scottish National Blood
Transfusion Service, SCOTLAND
Professor Ian M Franklin, Medical and Scientific Director,
Scottish National Blood Transfusion Service, SCOTLAND
Liz Pirie, Transfusion Education Specialist, Scottish National
Blood Transfusion Service, SCOTLAND
Advisory Board
Dr Dragoslav Domanic,
Director of the Blood Transfusion Centre of Slovenia
Professor Ian Franklin,
Medical and Scientific Director of the Scottish National Blood
Transfusion Service
Ms Lynda Hamlyn,
Chief Executive of NHS Blood and Transplant, UK
Mr Angus Macmillian, Douglas,
Former National Director of the Scottish National Blood
Transfusion Service
Prof. Dr. med. Dr. h. c. Erhard Seifried,
Ärztlicher Direktor und Medizinischer Geschäftsführer -
Instit für Transfusionsmedizin und Immunhämatologie Frankfurt a. M.
Project Team
Professor Ian Franklin
Medical and Scientific Director
Dr Brian McClelland
Consultant
Mrs Elizabeth Pirie
Transfusion Nurse Specialist, Better Blood Transfusion
Programme
Mrs Shirley Russell
Project Support Officer
11.2 References
General
Schramm WG ed. (1990) Blood safety in the European Community: an initiative for optimal use. ISBN 3-00-005705 European Commission
Schramm WG et al eds (2009) Report of European Symposium on Optimal Clinical Use of Blood Components April 24th-25th 2009, Wildbad Kreuth, Germany. In preparation
Directive 2002/98/EC of the European Parliament and of the Council of 27 January 2003 setting standards of quality and safety for the collection, testing, processing, storage and distribution of human blood and blood components and amending Directive 2001/83/EC
Council of Europe (2008) Guide to the preparation, use and quality assurance of blood components, 14th edition, ISBN 978-92-871-6330-1. Council of Europe Publishing
Scottish Intercolegiate Guidelines Network (2001) Perioperative Blood Transfusion for Elective Surgery, 54, Edinburgh
National Institute for Health and Clinical Excellence (2006)
‘The Guidelines Manual’, London, www.nice.org.uk
Smith, L.A. (2006) NHS Highland Clinical Governance Strategy and Risk Management, NHS Highland
Systematic reviews
Carless PA, Henry DA, Moxey AJ, O’Connell DL, Brown T, Fergusson DA. (2006) Cell salvage for minimising perioperative allogeneic blood transfusion Cochrane Database of Systematic Reviews Issue 4
Carson JL, Hill S, Carless P, Hebert P, Henry D. (2002) Transfusion triggers: a systematic review of the literature Transfusion Medicine Reviews Jul; 16,3,187-99
Hill SR, Carless PA, Henry DA, Carson JL, Hebert PC, McClelland DB, Henderson KM. (2000) Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion Cochrane Database of Systematic Reviews Issue 1
Stanworth SJ, Hyde C, Brunskill S, Murphy M. (2005) Platelet transfusion prophylaxis for patients with haematological malignancies: where to now? British Journal of Haematology 131, 5, 588-95
Stanworth SJ, Hyde C, Heddle N, Rebulla P, Brunskill S, Murphy MF. (2004) Prophylactic platelet transfusion for haemorrhage after chemotherapy and stem cell transplantation Cochrane Database of Systematic Reviews Issue 4
Stanworth SJ, Brunskill SJ Hyde CJ, McClelland DB, Murphy MF. (2004) Is fresh frozen plasma clinically effective? A systematic review of randomised controlled trials British Journal of Haematology 126, 1,139-52
Stanworth SJ, Brunskill SJ, Hyde CJ, Murphy MF, McClelland DBL. (2006) Appraisal of the evidence for the clinical use of FFP and plasma fractions Best Practice and Research Clinical Haematology, 19, 1, 67-82
Wilson J, Yao GL, Raftery J, Bohlius J, Brunskill S, Sandercock J, Bayliss S, Moss P, Stanworth S, Hyde C (2007) A systematic review and economic evaluation of epoetin alfa, epoetin beta and darbepoetin alfa in anaemia associated with cancer, especially that attributable to cancer treatment Health Technology Assessment Apr, 11, 13,1-220
Original papers
Auroy Y, Lienhart A, Péquignot F, Benhamou D. (2007) Complications related to blood transfusion in surgical patients: data from the French national survey on anesthesia-related deaths Transfusion Aug, 47 (2 Suppl): 184S-189S
Carson JL, Terrin ML, Magaziner J, Chaitman BR, Apple FS, Heck DA, Sanders D. (2006) FOCUS Investigators Transfusion trigger trial for functional outcomes in cardiovascular patients undergoing surgical hip fracture repair (FOCUS) Transfusion 46 (12): 2192-206
Hansen E., Knuechel, R., Altmeppen J., Taeger, K. (1999b) Blood irradiation for intraoperative autotransfusion in cancer surgery: demonstration of efficient elimination of contaminating tumor cells. Transfusion, 39, 608–614
Hébert PC, Wells G, Blajchman MA et al. (1999) With the Transfusion Requirements in Critical Care Investigators for the Canadian Critical Care Trials Group A multicentre, randomized controlled clinical trial of transfusion requirements in critical care New England Journal of Medicine 340: 409–17
Kirpalani H, Whyte RK, Andersen C, Asztalos EV, Heddle N, Blajchman MA, Peliowski A, Rios A, LaCorte M, Connelly R, Barrington K, Roberts RS. (2006) The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants Journal of Pediatrics Sep; 149, 3, 301-307
Lienhart A, Auroy Y, Péquignot F, Benhamou D, Warszawski J, Bovet M, Jougla E. (2006) Survey of anesthesia-related mortality in France Anesthesiology Dec; 105(6): 1087-97.
Mangano DT, Miao Y, Vuylsteke A, Tudor IC, Juneja R, Filipescu D, Hoeft A, Fontes ML, Hillel Z, Ott E, Titov T, Dietzel C, Levin J; (2007) Investigators of The Multicenter Study of Perioperative Ischemia Research Group; Ischemia Research and Education Foundation Mortality associated with aprotinin during 5 years following coronary artery bypass graft surgery JAMA Feb 7, 297, 5, 471-9.
McLellan, S.A., Walsh, T.S. and McClelland, D.B.L. (2002) Should we demand fresh red blood cells for perioperative and critically ill patients? British Journal of Anaesthesia, 89, 537-540 (Editorial) Rao SV, Jollis JG, Harrington RA, Granger CB, Newby LK, Armstrong PW, Moliterno DJ, Lindblad L, Pieper K, Topol EJ, Stamler JS, Califf RM. (2004) Relationship of blood transfusion and clinical outcomes in patients with acute coronary syndromes JAMA 292:1555-62.
SAFE Study Investigators (2004) Australian and New Zealand Intensive Care Society Clinical Trials Group; Australian Red Cross Blood Service; George Institute for International Health, Myburgh J, Cooper DJ, Finfer S, Bellomo R, Norton R, Bishop N, Kai Lo S, Vallance S. New England Journal of Medicine 350 2247-2246
Saline or albumin for fluid resuscitation in patients with traumatic brain injury New England Journal of Medicine 2007; 357,874-84
Sirchia, G., Giovanetti, A.M., Mc Clelland, B., Fracchia G.N. (eds.) (1994) Safe and good use of blood in surgery (SANGUIS) Use of blood products and artificial colloids in 43 European hospitals Report No. EUR 15398 EN, Office for Official Publications of the European Communities, Brussels/Luxembourg
Sanguis Study Group 91994) Use of blood products for elective surgery in 43 European hospitals Transfusion Medicine 4: 251–68.
The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. Journal of Pediatrics 2006 Sep; 149(3): 301-307
Thomas MJ. (1999) Infected and malignant fields are an absolute contraindication to intraoperative cell salvage: fact or fiction Transfusion Medicine 9, 269–278
Voak, Chapman and Phillips (2001) Quality of transfusion practice beyond the blood transfusion laboratory is essential to prevent ABO-incompatible death Transfusion 10, 2,95-96
Walsh TS, McArdle F, McLellan SA, Maciver C, Maginnis M, Prescott RJ, McClelland DB. (2004) Does the storage time of transfused red blood cells influence regional or global indexes of tissue oxygenation in anemic critically ill patients? Crit Care Med Feb; 32, 2,364-71.
Weiskopf RB, Feiner J, Hopf H, Lieberman J, Finlay HE, Quah C, Kramer JH, Bostrom A, Toy P. (2006) Fresh blood and aged stored blood are equally efficacious in immediately reversing anemia-induced brain oxygenation deficits in humans Anesthesiology May; 104, 5, 911
11.3 Links
International
World Health Organisation
Provides extensive resources on all aspects of transfusion. Resources for clinical transfusion practice improvement include:
-
The Clinical Use of Blood in Obstetrics, Paediatrics, Surgery & Anaesthesia, Trauma & Burns. (English, Portuguese, Spanish)
-
The Clinical Use of Blood Handbook (English, Portuguese, Spanish)
-
Recommendations on Developing a National Policy and Guidelines on the Clinical Use of Blood (English, Portuguese, Spanish, French).
http://www.who.int/bloodsafety/en/
International Haemovigilance Network: Resources include haemovigilance definitions, definitions, and downloadable papers on haemovigilance.
http://www.ihn-org.net/Portal.aspx
International committee of commonality in blood banking (ICCBBA) manages the ISBT 128 coding system. This is the global standard for the identification, labeling and information processing of human blood, tissue and organ products.
http://www.iccbba.org/
Europe
European Commission
European Commission Public Health Programme: In addition to the Eu Optimal Use of Blood project, the Commission supports the following projects
http://ec.europa.eu/health/index_en.htm
-
EU-Q-Blood SOP Project: Development of a pan-European standard operating procedure (SOP) methodology reflecting European best practice within the area addressing the quality and safety of blood.
http://www.eu-q-blood-sop.de/ -
European Blood Inspection System: Contains publications of this 'sister project', including Manual for Developing Standard Operating Procedures and Inspection Guide.
www.eubis-europe.eu/ -
Domaine:A collaborative project to develop resources for blood donor management in Europe. Includes donor recruitment strategies, donor retention strategies, deferral procedures and blood bank policy regarding patients requiring long-term transfusion
http://www.domaine-europe.eu/
Council of Europe: European Directorate for Quality of Medicines (EDQM): EDQM now hosts and manages the Council of Europe (programme on blood transfusion, including production of the Guide to the Preparation, Use and Quality Assurance of Blood Components. On the CoE site, search blood transfusion to access relevant pages. The 15the Edition of the Guide should become available on the website during 2010.
http://www.edqm.eu
Report on the collection, testing and use of blood and blood components in Europe in 2004
Haemovigilance Programmes of EU member states
- Denmark http://www.haemovigilance.dk/index_eng.htm
- France http://www.ints.fr
- Netherlands http://www.tripnet.nl/pages/nl/
- Luxembourg http://www.croixrouge.lu/
- Norway http://www.hemovigilans.no/
- Republic of Ireland http://www.giveblood.ie/Clinical_Services/Haemovigilance/
- Spain http://www.msc.es/profesionales/saludPublica/medicinaTransfusional/home.htm
- UK http://www.shotuk.org/
European Blood Alliance: Contributes to safety and security of the blood supply in Europe by developing and maintaining a network of partner blood services across Europe. Co sponsor of this Manual and website.
www.europeanbloodalliance.org
Eurocode (International Blood Labeling Systems): Website is currently uninformative
www.eurocode.org/content/index.html
German medical Association (Bundesaertzekammer): Includes the 2009 cross sectional guidelines for therapy with blood components and plasma derivatives.
http://www.bundesaerztekammer.de/
English language translation of the 2009 cross sectional guidelines for therapy with blood components and plasma derivatives:
http://www.bundesaerztekammer.de/downloads/LeitCrossBloodComponents4ed.pdf
UK Blood Services Professional Advisory Committee: Includes an evidence library with index of systematic reviews and randomised clinical trials relevant to transfusion. Toolkit of resources for quality improvement in transfusion practice and other resources, Handbook of transfusion medicine, Guidelines for the UK blood services.
http://www.transfusionguidelines.org.uk/
British Blood Transfusion Society: Provides information about education, professional development and training activities.
http://www.bbts.org.uk/
British Committee for Standards in Haematology: Displays UK Guidelines for transfusion and other aspects of haematology.
http://www.bcshguidelines.com/
National Institute for Clinical Excellence in the UK: Provides national evidenced based guidance for health professionals. Tools to support service redesign and implementation of NICE guidance.
http://www.nice.org.uk/
National Institute for Health Centre Graduate Medical Education: Training Programs: Has developed a comprehensive portfolio of clinical research training and medical education initiatives.
http://www.cc.nih.gov/index.html
National Patient Safety Agency: Provides a toolkit of resources to assist with improving patient safety. Has information on safe patient identification and competency assessment for blood transfusion.
http://www.npsa.nhs.uk/
NHS Quality Improvement Scotland:An organisation which asists NHS hospitals in Scotland to improve patient care by setting standards. Assesses and measures the performance of hospitals against these standards. Standards for blood transfusion and the peer review inspection reports are available.
http://www.nhshealthquality.org/
Network for the Advancement of Transfusion Alternatives
Provides information about alternatives to transfusion.
http://www.nataonline.com/
Scottish Intercollegiate Guidelines Network (SIGN)
Developed an evidence-based guideline, which aims to maximise patient safety by helping clinicians decide when transfusion is appropriate.
http://www.sign.ac.uk/
Scottish National Blood Transfusion Service
Better Blood Transfusion -continuing education programme
An elearning programme designed at three levels. Targets all practitioners involved in the clinical transfusion process.
http://www.learnbloodtransfusion.org.uk/
Skills for Health
Provides resources to assists the UK health sector develop a skilled workforce e.g. career pathways, competencies.
http://www.skillsforhealth.org.uk/
Centre for Maternal and Child Enquiries
The Centre for Maternal and Child Enquiries' mission is to improve the health of mothers, babies and children by carrying out confidential enquiries on a UK wide basis.
- Maternal death enquiry
- National perinatal and maternal mortality surveillance
- Maternal and perinatal enquiry projects
- Child health enquiry projects
Australia
Australian Blood Services
Gives health professionals access to information about blood and blood products, from donation to transfusion and beyond. It includes a Transfusion Medicine Manual, which contains more detailed information about blood products and clinical practice. Circular of information about blood components (2009), links to e- learning sites and a variety of downloadable resources.
http://www.transfusion.com.au/home.aspx
Canada
Canadian Transfusion Safety Officers
Provides access to clinical and technical guidelines and a variety of downloadable resources.
http://www.transfusionsafety.ca/
Canadian Institutes for Health Research and the Heart and Stroke Foundation of Canada: Offers training in blood transfusion science. http://www.cbr.ubc.ca/STPTS.htm
TraQ Program of the British Columbia Provincial Blood Coordinating Office
Focuses on quality improvement among transfusion medicine professionals through timely sharing of educational resources related to current best practices and guidelines in transfusion medicine. Website offers multiple educational tools, including a resource library, interactive case studies, regulatory information, Canadian, international, industry and government news, job postings, events calendar, patient resources, and more.
http://www.TraQprogram.ca/
Report of the Krever Inquiry into the blood system in Canada.
Provides an extensive account of responses to HIV and Hepatitis C in relation to transfusion in Canada and internationally
www.hc-sc.gc.ca/ahc-asc/activit/com/krever_e.html
USA
Harvard Medical School Fellowship Program in Transfusion Medicine
Offers a core curriculum for blood transfusion. http://www.harvardtransfusion.org/curriculum.pdf
The Joint Commission (Health Care Accreditation)
Accredits and certifies more than 16,000 health care organizations and programs in the United States. 34 www.jointcommission.org/