Why optimal blood use is important
The safety of hospital treatment and the effectiveness of care are major concerns in healthcare systems. Blood transfusion has been the subject of legal proceedings and investigations in Canada, England, France, Ireland and other countries. Hospitals should be in a position to show that their practice of blood transfusion is safe, clinically effective and efficient. Specific reasons for this are as follows.
Accountability
Blood is a human tissue and is a precious and scarce resource. Many countries have difficulties matching supply with demand. The supply of blood components in the EU depends substantially on the support of voluntary donors. Both the ageing population in many EU countries and the effect of new precautionary measures to safeguard blood recipients have increased the problems of maintaining a sufficient supply of blood. Transfusion services promote donation as an essential contribution to the care of patients, so both hospitals and blood collection services have an obligation to demonstrate to blood donors that each gift of human tissue is carefully, wisely and effectively used and that it can be fully accounted for. Patients need assurance that blood is safe, available and used only when required.
Compliance with EU legislation
EU Blood Directives place a legal responsibility on hospital managements to introduce a quality system to important parts of the transfusion chain. Blood establishments are required, to maintain quality management systems and to undergo regular inspection.
Hospital blood banks must submit an annual compliance form and may be inspected on the basis of this return. The reporting of adverse events is a legal requirement in the EU as is the ability to trace every blood component from its donor to the patient who receives it.
Accreditation
Institutions that seek accreditation by bodies such as the Joint Commission or the Care Quality Commission in the UK will need to show evidence of a quality management system.
R42 is downloadable from files at the end of the page.
www.nhshealthquality.org
Legal and media pressures
Legal actions, public inquiries, investigations or adverse media attention stimulated by transfusion-related harm to patients are likely to gain serious management attention (and the application of resources) to avoid future problems. Experience in several countries has shown that adverse events can cause medico-legal, publicity and reputational risks for a hospital and sometimes for the wider healthcare system.
Cost
The cost of providing blood components has increased as a result of new safety requirements and other technical developments. As an example in France, the overall cost of blood components increased by 37% between 1998 and 2008. The cost per inhabitant of France was €6.8 in 1999 and €8.8 in 2008.
Summary of the manual
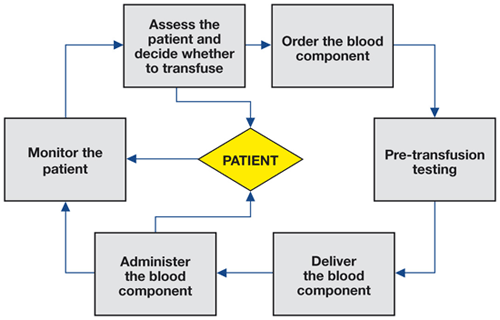
Details of the clinical transfusion process vary among the member states of the EU, but there are essential steps that are common to most. These are shown in Figure 1.1, and more detail is shown in the purpose section of this site (figure 2.1).
Quality improvement: analysis and prevention of errors
The following figures (1.2 to 1.7) share the same layout and show, for each of the main steps in the clinical transfusion process, examples of errors or failures in the process, the possible consequences for the patient, some underlying reasons for failures or errors, and finally some key points about prevention and avoidance. The subject matter of these tables is covered in more detail in the various sections throughout the site.
Figure 1.2 Analysis and prevention of errors in clinical transfusion decisions
|
Clinical Decision
|
| |
| Steps in the process |
What can go wrong |
Consequences
for the patient |
Why it goes wrong |
Prevention
and avoidance |
- Assess clinical condition
- Decide if transfusion indicated, which component and number of units
- Discuss with patient
- Obtain consent
- Record indication for transfusion and the discussion with patient
|
- Wrong clinical decision
- Unnecessary transfusion
- Failure to give a necessary transfusion
- Wrong component given
- Wrong dose given
- Patient not informed
- Decision not recorded
- Patient case record lost
|
- Transfusion associated circulatory overload
- Avoidable exposure to infection or immunological risk
- Risk of myocardial ischemia
- Patient makes complaint
- No record available to defend medicolegal challenge
|
- Lack of transfusion knowledge or failure to follow guidelines
- Inadequate clinical assessment
- Unaware of importance of information and consent
- No patient information available
- Information given at wrong time
- Patient couldn’t read or understand information
|
- Clinical guidelines are available
- Compliance with guidelines is audited
- Prescriber has a thorough knowledge of the indications for blood components and the knowledge to answer patient’s questions
- Written patient information is provided, at right time and is legible and understandable
- Consent should be recorded
- Compliance with procedures is audited
- Errors, events and reactions are investigated
- Procedures improved by lessons learned
|
Figure 1.3 Analysis and prevention of errors in ordering blood components
|
Patient Sample and Request for Blood
|
| |
| Steps in the process |
What can go wrong |
Consequences
for the patient |
Why it goes wrong |
Prevention
and avoidance |
- Identify patient correctly
- Decide which component is needed and the quantity
- Complete blood request form or electronic order
- Take pre-transfusion sample
- Send blood sample and request to hospital blood bank
- If required, initiate major haemorrhage procedure (MHP)
|
- Pretransfusion sample taken from wrong patient
- Failure to communicate transfusion requirements
- Incorrect blood group in patient’s record
- Inappropiate dose/volume
- Patient receives blood intended for another person
- Failure to recognise a major haemorrhage
- Major haemorrhage procedure not activated
|
- Immunosuppressed patient put at risk of graft versus host disease
- Delayed haemolytic transfusion reaction
- Young female sensitised to RhD
- Patient transfused with wrong component or quantity
- Fatal ABO incompatibility reaction
- Death or serious complications due to delayed transfusion
|
- Inadequate information on form
- Request form completed incorrectly
- Incorrect details on sample tube
- Correct patient, but sample tube wrongly labelled
- Sample taken from wrong patient
- Sample transport inappropriate for situation
- Ignorance of major harmorrhage procedure (MHP)
- No MHP available
|
- Patient identification policy in place and observed
- Minimum data set for patient ID in place and observed
- Prescriber knows procedure for pretransfusion sample and blood request
- Prescriber knows the indications for particular type of component (e.g. irradiated), establishes patient’s requirement and orders correctly
- Clinical laboratory and transport staff are familiar with and trained in major haemorrhage protocol
- MHP is practised periodically (“fire drill”)
- Compliance with procedures is audited
- Errors, events and reactions are investigated
- Procedures improved by lessons learned
|
Figure 1.4 Analysis and prevention of errors in pretransfusion testing
|
Errors in Pretransfusion Testing
|
| |
| Steps in the process |
What can go wrong |
Consequences
for the patient |
Why it goes wrong |
Prevention
and avoidance |
- Note urgency of request.
If necessary, confirm with requesting clinician
- Select approved procedure that is suitable for degree of urgency
- When request is received check (control) patient sample and request for consistency and completeness of patient ID data
- Note any specific requirements (e.g. irradiated component)
- Determine patient’s ABO and RhD type. Screen patient’s serum for red cell alloantibodies
- Check if this data is consistent with any previous laboratory records for the patient
- Select suitable units
- Perform compatibility test (crossmatch)
- Label, record and dispatch selected units
|
- Urgency misunderstood
- Inappropriate procedure selected
- Patient sample and request not checked for consistency and completeness
- Verbal correction of details accepted
- Requesting clinician does not specify
- Blood bank staff do not register the requirement
- Blood bank records not checked
- Error in testing procedure or recording of results
- Failure to check if previous record exists or to find record
- Failure to select appropriate units (e.g. irradiated)
- Error in testing procedure or recording of results
- Incorrect labelling
- Dispatch to wrong destination. Inappropriate transport method
|
- Delayed transfusion: risk of exsanguination
- Risk of incompatible transfusion due to mistaken identification
- Delayed transfusion
- RhD sensitisation of Rh O negative recipient
- Delayed haemolytic reaction due to missed alloantibody
- Risk of graft versus host disease
|
- Failure of communication
- Blood bank reluctance to issue uncrossmatched red cells
- Clinical unit reluctant to transfuse uncrossmatched red cells
- Staff failure to comply with SOP
- Poor training
- No SOP
- Failure by requesting clinical staff
- Defective or lost patient records
- Defective reagents
- Defective equipment
- Inadequate records system in blood bank
- Suitable units not available
|
- Major haemorrhage procedure should specify how urgent requests are communicated
- Blood bank should insist on correct identification and a fresh sample if necessary
- Training for all staff concerned
- Internal and External Quality control of blood bank performance
- Install effective paper or computerised system staff training
- Maintain appropriate stock in blood bank
|
Figure 1.5 Analysis and prevention of errors in delivering blood to the clinical area
|
Deliver Blood Component to the Clinical Area
|
| |
| Steps in the process |
What can go wrong |
Consequences
for the patient |
Why it goes wrong |
Prevention
and avoidance |
- Pick up blood component from storage site
- Deliver blood component promptly to clinical area
- Blood component received in clinical area
- Store correctly until transfused
|
- Wrong unit selected
- One or more patients receive an incorrect blood component
- Delay in supplying blood
- Blood delivered to wrong location
- Blood discarded because of incorrect storage
- Wrong storage e.g. placed in freezer or left on heater
|
- Fatal or serious haemolytic transfusion reaction
- Delayed haemolytic transfusion
- Uncorrected severe anaemia
- Increased risk of transfusion to wrong person
- Blood units wasted
- Transfusion reaction due to contaminated or thermally-damaged blood
|
- Written patient details not used to select blood unit from storage
- Delivered to wrong location
- Clinic staff unaware that blood delivered
- Blood component damaged by incorrect temperature storage
|
- Take written patient ID details when collecting blood units
- Staff responsible for collecting blood are trained in correct procedures
- Standard procedures are documented
- Compliance with procedures are audited
- Errors, events and reactions are investigated
- Procedures improved by lessons learned
|
Figure 1.6 Analysis and prevention of errors in administering (transfusing) blood
|
Administer Blood Component
|
| |
| Steps in the process |
What can go wrong |
Consequences
for the patient |
Why it goes wrong |
Prevention
and avoidance |
- Check patient identity details
- Check written prescription
- Ensure IV line is in order
- Take base line observations
- Inspect condition of unit
- Check expiry date
- Check that patient ID details on identification band and blood component match
- Check that ABO and RhD group on the patient ID label and blood component label match
- Start transfusion at flow rate instructed
|
- Transfusion delayed
- Contaminated pack not detected
- Outdated pack transfused
- Patient receives incorrect blood component
- Component transfused too quickly
- Transfusion details not documented
|
- Transfusion-associated sepsis
- Death due to transfusion of contaminated unit
- Morbidity due to transfusion of partially haemolysed unit (past its expiry date)
- Death due to ABO incompatibility reaction
- Volume overload (TACO)
- Unit not traceable
|
- Pack not inspected
- Discoloration or change in component not noticed
- Expired pack not identified
- Check of patient and unit not performed
- Instructions for infusion not clear or not followed
- Failure to adhere to standard procedure
|
- Patient identification policy in place and observed: effective “bedside” check
- Minimum data set for patient ID in place and observed
- Staff responsible for administering blood transfusions trained in procedure
- Standard procedures are documented
- Compliance with procedures are audited
- Errors, events and reactions are investigated
- Procedures improved by lessons learned
- Computerised support system
|
Figure 1.7 Analysis and prevention of errors in monitoring the transfused patient
|
Monitor the Transfused Patient
|
| |
| Steps in the process |
What can go wrong |
Consequences
for the patient |
Why it goes wrong |
Prevention
and avoidance |
- Observe patient’s condition and vital signs
- Recognise and respond appropriately to adverse event
- Record outcome of transfusion
- Assess need for further transfusion
|
- Adverse reaction not detected
- Adverse reation not managed correctly
- Delay in obtaining medical assistance
- Delay in assessing continued transfusion requirement
|
- Avoidable harm to patient
- Delayed response to transfusion reaction
- Major morbidity or death due to transfusion event
- Incomplete follow-up or investigation
- Records inadequate should there be a complaint or legal challenge
|
- Patient not monitored
- Adverse reaction not recognised
- Adverse reaction not responded to appropriately
- Clinical help not called for
- Clinician called fails to respond
- Clinician does not treat the patient’s reaction correctly
|
- Doctors and nurses responsible for transfused patients are trained in management of adverse reactions
- Clinical guidelines for management of adverse reactions are available and are used
- Adverse reactions are investigated
- Procedures improved by lessons learned
|

